The 10 types of chemical bonds (explained with examples)
Chemical bonds are the forces that hold atoms together to form the molecules. There are three types of bonds between atoms:
- Metallic bond.
- Ionic bond.
- Covalent bond: nonpolar, polar, simple, double, triple, dative.
Thanks to these bonds, all the compounds that exist in nature are formed. There are also forces that hold molecules together, known as intermolecular bonds, such as:
- Hydrogen bonds or bridges.
- Dipole-dipole forces.
Next, we explain each of these links.
| Types of chemical bond | Characteristic | Examples | |
|---|---|---|---|
| Metal | Metal ions float in a sea of moving electrons. | Metallic elements: sodium, barium, silver, iron, copper. | |
| Ionic | Transfer of electrons from one atom to another. | Na sodium chloride+Cl- | |
| Covalent | Non polar | Share electrons equally between two atoms. | Molecular hydrogen H-H or H2 |
| Polar | Shares electrons unevenly between two atoms. | Water molecule H2OR | |
| Simple | Share a pair of electrons. | Chlorine molecule Cl2 Cl-Cl | |
| Double | Share two pairs of electrons. | Oxygen molecule O2 O = O | |
| Triple | Share three pairs of electrons. | Nitrogen molecule N≣N or N2 | |
| Dative | Only one of the atoms shares the electrons. | Bond between nitrogen and boron in the compound ammonia-boron trifluoride. | |
| Intermolecular forces | Hydrogen bridge | The hydrogens in one molecule are attracted to the electronegative atoms of another molecule. | Hydrogen bonds between the hydrogen in one water molecule with the oxygen in another water molecule. |
| Dipole-dipole | Molecules with two electrical poles attract the opposite poles of other molecules. | Interactions between methanal H molecules2C = O |
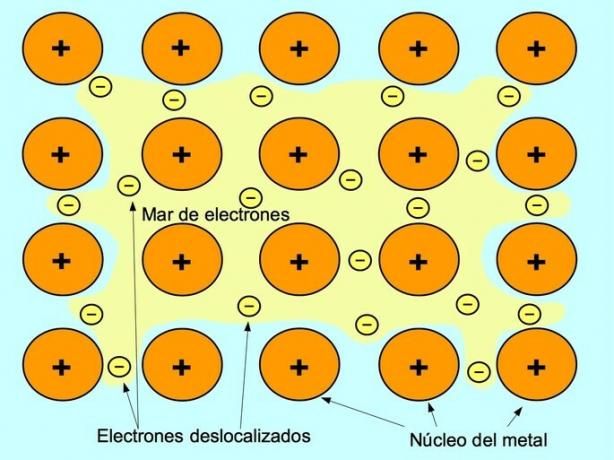
Metallic bond
The metallic bond is the force of attraction between the positive ions of the metallic elements and the negative electrons that are free moving between the ions. Metal atoms are tightly packed, this allows electrons to move within the lattice of atoms.
In metals, the valence electrons are released from their original atom and form a "sea" of electrons that floats around the entire metal structure. This causes the metal atoms to transform into positively charged metal ions that pack together.
The metallic bond is established between metallic elements such as sodium Na, barium Ba, calcium Ca, magnesium Mg, gold Au, silver Ag and aluminum Al.
Ionic bond
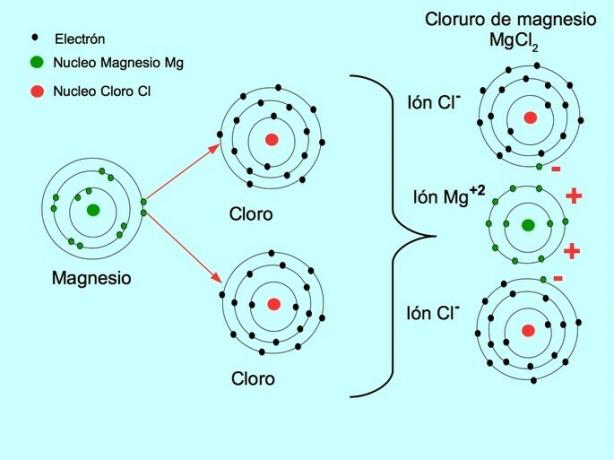
The ionic bond is the force that joins a metallic element, such as sodium or magnesium, with a non-metallic element, such as chlorine or sulfur. Metal loses electrons and transforms into a positive metal ion called cation. These electrons pass to the non-metallic element and it transforms into a negatively charged ion called anion.
The cations and anions combine and form a three-dimensional network that is maintained by the forces of electrostatic attraction between the ions with different charges. These forces form ionic compounds.
The earth's crust is made up mainly of ionic compounds. Most rocks, minerals, and gemstones are ionic compounds. For example:
- Sodium Chloride NaCl: the metallic element is sodium that transfers an electron to chlorine, which is the non-metallic element.
- Magnesium Chloride MgCl2: Magnesium Mg donates two electrons to two chlorine atoms, as shown in the figure below:
See also Difference between cations and anions.
Covalent bond
The covalent bond forms when two non-metallic atoms share electrons. This bond can be of several types depending on the affinity for the electrons of the atoms and the amount of shared electrons.
Nonpolar covalent bond
The nonpolar covalent bond is the bond that forms between two atoms where electrons are shared equally. This bond normally occurs in symmetric molecules, that is, molecules made up of two equal atoms, such as the hydrogen molecule H2 and the oxygen molecule O2.

Polar covalent bond
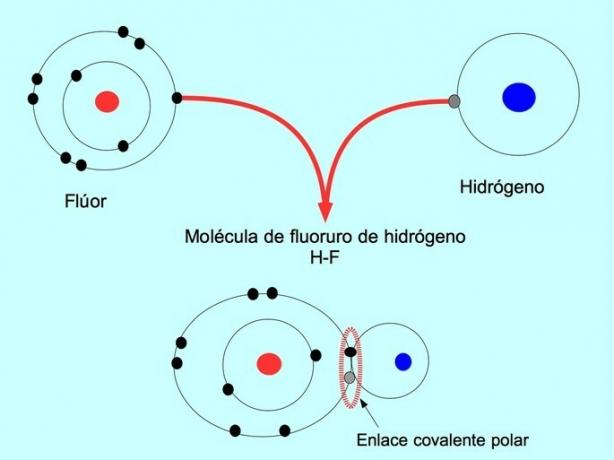
The polar covalent bond is formed when two atoms share electrons but one of them has a greater attraction for electrons. This makes the molecule have a more negative "pole" with more electrons and the opposite pole is more positive.
Molecules with this distribution or imbalance of electrons are known as polar. For example, in hydrogen fluoride HF, there is a covalent bond between hydrogen and fluorine, but fluorine has higher electronegativity, so it attracts electrons more strongly shared.
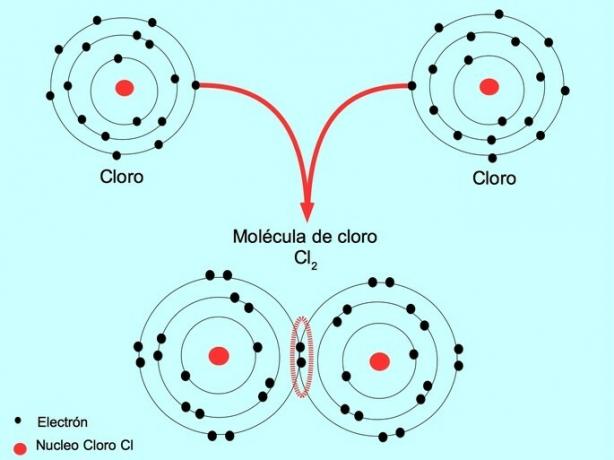
Simple covalent bond
When two atoms share two electrons, one from each, the covalent bond formed is called a single covalent bond.
For example, chlorine is an atom that has seven valence electrons in its outer shell, which can be filled with eight electrons. A chlorine can combine with another chlorine to form the chlorine molecule Cl2 which is much more stable than chlorines alone.
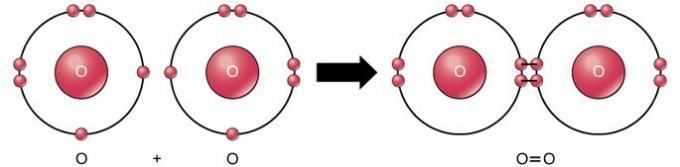
Double covalent bond
The double covalent bond is the bond where four electrons (two pairs) of electrons are shared between two atoms. For example, oxygen has 6 electrons in its last shell. When two oxygens are combined, four electrons are shared between the two, causing each to have 8 electrons in the last shell.
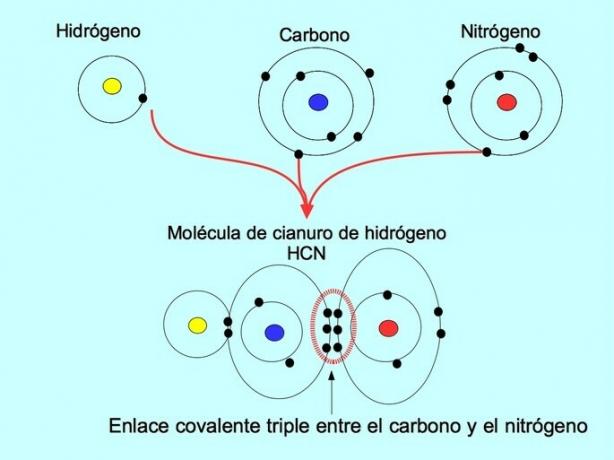
Triple covalent bond
The triple covalent bond is formed when 6 electrons (or three pairs) are shared between two atoms. For example, in the hydrogen cyanide molecule HCN, a triple bond is formed between carbon and nitrogen, as presented in the figure below:
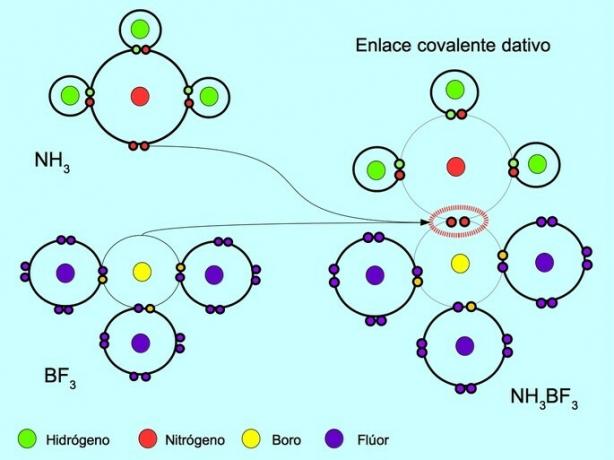
Coordinate or dative covalent bond
The coordinated or dative covalent bond is the bond that forms when only one of the atoms in the bond contributes an electron pair. For example, when ammonia NH reacts3 with boron trifluoride BF3, Nitrogen bonds with two electrons directly to boron, which has no electrons available to share. In this way, both nitrogen and boron are left with 8 electrons in their valence shell.
See also Organic and inorganic compounds.
Intermolecular links
Molecules associate through forces that make it possible to form substances in liquid or solid state.
Dipole-dipole bonds or forces
Weak intermolecular bonds can be established between polar molecules when negative poles are attracted to positive poles and vice versa. For example, methanal H2C = O is a polar molecule, with a partial negative charge on oxygen and a partial positive charge on hydrogens. The positive side of one methanal molecule attracts the negative side of another methanal molecule.
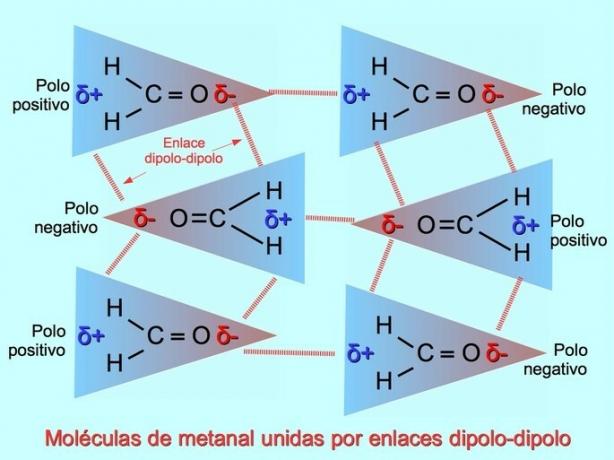
Hydrogen bonds or bonds
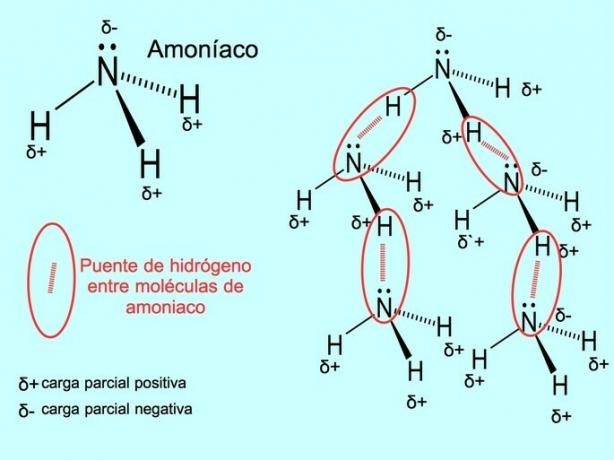
The hydrogen bond or hydrogen bond is a bond that is established between molecules. It occurs when a hydrogen in the molecule is covalently bound to an oxygen, a nitrogen or a fluorine. Oxygen, nitrogen and fluorine are atoms with higher electronegativity, therefore they attract electrons more strongly when they share them with another less electronegative atom.
There are hydrogen bonds between water molecules H2O and ammonia NH3 as the picture shows:
You may be interested in seeing:
- Atoms and molecules.
- Examples of organic and inorganic compounds.
- Metals and non-metals
References
Zumdahl, S.S., Zumdahl, S.A. (2014) Chemistry. Ninth Edition. Brooks / Cole. Belmont.
Commons, C., Commons, P. (2016) Heinemann Chemistry 1. 5th edition. Pearson Australia. Melbourne.