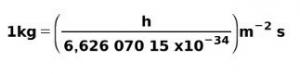Difference between physical and chemical properties of matter
The physical properties of matter are those characteristics that can be measured without altering the atomic structure, while chemical properties of matter are those characteristics that result in a change in the atomic structure.
| Physical properties | Chemical properties | |
|---|---|---|
| Definition | Characteristics of matter that can be measured without changing its chemical formula. | Characteristics of matter where molecular change occurs. |
| Chemical formula | It does not change | Change |
| Reversibility | Reversible | Irreversible |
| Dependence on the amount of matter |
|
Independent of the amount of matter |
| Examples |
Mass Volume Density Boiling point Melting point Crystal configuration |
Heat of combustion Reactivity Electronegativity Ionization |
Physical properties of matter
The physical properties of matter are those characteristics that can be measured and observed without the substance changing its chemical formula.
Examples of physical properties
Here are some physical properties of matter with examples.
Mass
Mass is the physical property that expresses the amount of matter that a body contains. In physics, mass is defined as the measure of an object's resistance to acceleration. The units of measurement are the gram and its multiples. For example, 1 kilogram of iron, 10 grams of gold, or 0.1 milligrams of glucose.
Volume
Volume is the measure of the space that a substance or body occupies. The units of measurement are the liter and its multiples. For example, 1 liter of milk, 500 milliliters of water, or 5 microliters of mercury.
Density
Density is the ratio of the mass to the volume of a body. For example, aluminum has a density of 2.7 grams / ml, that is, 1 ml of aluminum has a mass of 2.7 grams.
Temperature
Temperature is the measure of the internal agitation of a system. It is measured with the help of a thermometer and different scales are used: Celsius, Kelvin or Fahrenheit.
Electric resistance
Electrical resistance is an electrical physical property that determines how difficult it is for current to flow through a material. For example, silver, copper, and aluminum have low electrical resistance, while glass, rubber, and wood have high resistance to current.
Boiling point
The boiling point is the temperature at which a substance goes from a liquid state to a gaseous state. For example, ethanol (ethyl alcohol) goes from its liquid to gaseous state at a temperature of 78.37 ºC.
You may also be interested in seeing:
- Intensive and extensive properties
- States of matter and properties of matter.
Chemical properties of matter
The chemical properties of matter are those characteristics that are manifested when there is a change in the chemical structure of matter. That is, in order to measure this property, the substance reacts and changes its chemical constitution.
Examples of chemical properties
Here are some chemical properties of matter with examples.
Heat of combustion
Heat of combustion is the energy that is released when a substance is burned. Combustion is the reaction of a substance with oxygen. For example, in the combustion of one mole of methane (CH4) 213 kcal is released.
Reactivity
Reactivity is the property of a substance to react with another substance. For example, oxygen is one of the most reactive elements in the universe, while neon is one of the least reactive elements.
Affinity for electrons
The affinity for electrons of an atom or molecule is the property of gaining electrons. For example, chlorine Cl has a higher affinity for gaining an electron than sodium Na.
Ionization
Ionization is the property of an atom or molecule to form ions, a species that is electrically charged by the gain or loss of electrons. For example, hydrochloric acid HCl in aqueous solution ionizes to form the chloride anion Cl- and the hydronium cation H3OR+.
You may be interested in seeing:
- Elements, compounds and mixtures
- Physical and chemical phenomena.

Doctor in Biochemistry from the Venezuelan Institute of Scientific Research (IVIC), with a degree in Bioanalysis from the Central University of Venezuela.