Characteristics of acids and bases: main differences
The characteristics of acids and bases are all those properties and qualities with which we can distinguish between an acid and a base.
According to Arrehnius' theory, a acid is a substance that releases protons H+ when dissolved in water, and a base is any substance that releases hydroxyl ions OH- when in aqueous solution.
| Acids | Bases | |
|---|---|---|
| Ion formation | Present | Present |
| Neutralization reaction | When it reacts with a base. | When it reacts with an acid. |
| Water solubility | Soluble | Partially soluble. Calcium, barium and aluminum hydroxides are poorly soluble. |
| Electric conductivity | Present | Present |
| State of matter | Solid, liquid or gaseous | Solid, liquid or gaseous |
| Reaction with litmus paper | Red coloring | Blue coloring |
| PH value | Under 7 | Greater than 7 |
| Conjugate counterpart | A weak acid forms a conjugate base. | A weak base forms a conjugated acid. |
| Corrosive effect | Strong acids | Strong bases |
Characteristics of acids
Acids have several characteristics with which they can be identified.
Ability to form ions from acids
The main characteristic of acidic substances is that they can be ionized, that is, lose or gain electrons.
Hydrochloric acid HCl, a strong acid, ionizes to form chloride anion Cl- and proton H+.
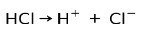
Neutralization reaction
An acid reacts with a base to form a salt and water. This reaction is known as neutralization reaction. For example, HCl reacts with NaOH to form sodium chloride NaCl (kitchen salt) and water:
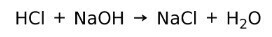
Water solubility of acids
Acids are generally soluble in water. For example, hydrochloric acid or muriatic acid is moderately soluble in water, up to 82 g of HCl can be dissolved in 100 ml of water at 0ºC. In the case of sulfuric acid H2SW4, the mixture with water generates heat, so it is always advisable to add the acid to the water to avoid explosions.
Acid strength
How acids dissociate determines whether they are strong or weak. The strength of an acid is given by its dissociation constant.
Nitric acid HNO3 it's a strong acid because in aqueous solution it completely ionizes into protons and nitrate ions:

This means that when we add HNO3 in water, when analyzing the water we will only find H+ and nitrate ions, and virtually no HNO3.
Instead, the acetic acid found in vinegar is a weak acid, because only part of it is dissociated:
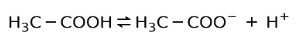
That is why it is represented with arrows in two directions, this means that in aqueous solution we will have acetic acid, H+ and the acetate anion (H3C-COO-).
States of matter of acids
Acids can be found in a liquid, solid or gaseous state. For example, hydrochloric acid HCl is liquid, hydrogen sulfide HS is a gas, and oxalic acid is a solid.
Reaction with litmus paper from acids

When we place a strip of litmus paper in an acidic substance, the litmus paper turns red.
Identification of acids by their pH
PH is the measure of the amount of H+ present in a solution. Thus, between pH 0 and 7, we are in the presence of an acid. For example, vinegar has a pH equal to 2, the pH of sulfuric acid in car batteries is equal to 1.
Electrical conductivity of acids
Since acids can dissociate and produce electrically charged ions, acidic solutions can conduct electricity. For example, inside car batteries, sulfuric acid, which is a strong acid, is used as a conductor of electricity.
Corrosive effect of strong acids
Strong acids have a corrosive effect, they can burn organic tissues so their handling must be extremely careful.
Weak acids form a conjugated counterpart
When dissolved, weak acids form what is known as a conjugate pair, that is, a weak acid forms a conjugate base.
For example:

In this case, formic or methanoic acid (HCOOH), when dissociated, forms the conjugated base ion format.
It may interest you:
- Strong and weak acids and bases.
- Examples of acids and bases.
Characteristics of the bases
Next we present the properties of the bases or alkaline solutions that characterize them.
Base ionization capacity
Basic or alkaline substances are characterized by forming ions when dissolved in water:

In this case, the sodium hydroxide NaOH ionizes to form a hydroxide anion OH- and a sodium cation Na+.
A base reacts with an acid to form a salt
A base reacts with an acid in a neutralization reaction to form water and a salt, for example:

In this case, the aluminum hydroxide Al (OH)3, a base, reacts with HCl and forms the aluminum chloride salt AlCl3 and water. Aluminum hydroxide is used as an antacid to relieve gastric reflux by neutralizing the acid produced by the stomach.
Bases water solubility
Some bases are soluble in water. Alkaline earth hydroxides, such as calcium hydroxide and barium hydroxide, are poorly soluble in water. For example, sodium hydroxide or caustic soda can be dissolved 109 g in 100 ml of water at 20ºC. While magnesium hydroxide or milk of magnesia Mg (OH)2 it is practically insoluble in water.
Base strength
Depending on the degree of ionization of the base, these can be strong or weak. For example, lithium hydroxide is a strong base because in aqueous solution it completely ionizes into hydroxide ions OH and lithium Li cations+:
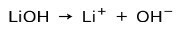
On the other hand, ammonia NH3 is a weak base because when it comes into contact with water, not all the ammonia dissociates:
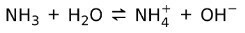
State of Matter of the Bases
Bases can be found in solid, liquid and gaseous states. For example, pure sodium hydroxide is solid, ammonia is a gas.
Reaction with the litmus paper of the bases
When we place a strip of litmus paper in an alkaline substance, the paper turns blue.
Identification of bases by their pH
The bases are characterized by having a pH between 7 and 14.
 Electrical conductivity of the bases
Electrical conductivity of the bases
The bases due to their ionization capacity are good conductors of electricity. For example, in alkaline batteries, potassium hydroxide KOH is used as the electrical conductor.
Corrosive effect of bases
Strong bases can damage organic tissues. For example, kitchen oven cleaners are generally strong bases, and it is always recommended to handle them with extreme care, wearing gloves and eye protection.
Weak bases form a conjugate counterpart
The weak bases when dissolved form a conjugated counterpart, that is, a weak base forms a conjugated acid.
For example, tris- (hydroxymethyl) amino methane (OHCH2)3CNH2 is a weak base whose conjugate acid is (OHCH2)3CNH3+:
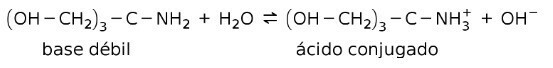
This is the basis for the action of buffer substances or buffer, which are substances that are used to maintain the pH of solutions constantly.
You may be interested in knowing the differences between:
- Acids and bases.
- Cations and anions