Electrostatic pressure: what is it and what are its characteristics
The world of electricity is exciting. From the operation of a battery to the emission of neurons within the human body, this set of Physical phenomena related to the presence and flow of charges allow us, as living beings, to think, move and exist.
On a social level, electricity has also provided us with an inestimable amount of resources: transportation, lighting, air conditioning and computing, which is soon to be said.
It is very curious to know that all living cells in our body have their own electrical charge. As the concentration of salts is different in the intracellular and extracellular environments (calcium, chlorine, sodium, potassium, etc.) an electric charge and potential difference are established between both media, a term known as " membrane".
The variation in the potentials of membranes in body cells allows us to think (electrical synapse at the neuronal level) to contract a voluntary muscle, due to the transmission of action potentials and hyperpolarization or depolarization in each process specific. As you can see, electricity goes far beyond a battery: stay with us and
find out everything about electrostatic pressure.- Related article: "Transcranial electrical stimulation: definition and applications"
What are the basics of electrostatics?
Electrostatics is defined as that branch of science that studies the mutual effects that occur between bodies as a result of their electrical charges.. All objects on Earth are made up of atoms, the smallest constituent units of matter with the properties of a chemical element. At rest, the positive charges of the atomic nucleus (99.94% of the total weight) balance with the negative charges of the surrounding electrons, so the object is considered to be at rest.
When an atom loses or gains electrons, it acquires a positive or negative electrical charge. By common convention, when an atom loses one or more electrons it is considered "positively charged" (since protons are charged positive and they are more in number than the negative electrons), whereas if the atom integrates electrons, it happens to have a negative charge. From here, both are called ions, whether they are positive or negative.
When an atom or molecule acquires a charge, it automatically becomes influenced by electromagnetic fields and generates them by itself.. Based on this premise, we can describe many biological phenomena, such as chemical bonds. For example, the ionic bond, which consists of the transmission of electrons from a metallic atom (less electronegative) to non-metallic ones (more electronegative).
What is electrostatic pressure?
Entering flour, we fear that we cannot give you a very exact definition of this term, as it seems to be slightly out of use in the scientific community. Various portals use the word "electrostatic pressure" to designate the electrical force of attraction or repulsion between particles with different or identical electrical charge, respectively.
If we embrace this term, we will see that the most correct to refer to this electrostatic phenomenon is "electric force". The electric force or electrostatic pressure will then be the force that appears between two or more charges, whose modulus depends on the value of the charges and the distance that separates them (and the sign depends on each load). This terminological conglomerate can be summarized in the following points:
- Charged atoms or molecules suffer a force of attraction or repulsion when approaching. Two ions with the same charge repel each other, but if one is positive (+) and the other negative (-) they get closer.
- The value of the electrostatic force or pressure is proportional to the product of the value of its charges.
- On the other hand, the value of this force is inversely proportional to the square of the distance that separates the charged atoms and acts in the direction of the line that joins them.
Today, These postulations settled in the field of physics are included under the umbrella of Coulomb's Law, enunciated by the French physicist Charles-Augustin de Coulomb in the year 1785. These applications can be collected in the following formula:
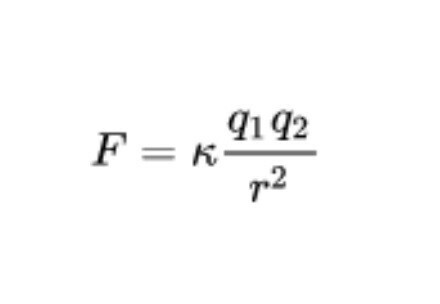
In this formula, F refers to the total electric force or electrostatic pressure, k is the Coulomb constant, q1 and q2 are the values of the charges of the atoms mentioned (in coulombs) and r the distance between both charges in meters at square. As a note, it should be noted that the unit "coulomb" or "coulomb" is defined as the amount of charge carried in one second by a current of one ampere of electrical current intensity.
The desired result (F) represents the attractive or repulsive force in Newtons between both electrically charged atoms or molecules.. The electric force or electrostatic pressure is a vector quantity, so, in addition to calculating the module, its direction and direction must also be estimated. If we only have two atoms in play, the direction of the electric force will be in line with the line that joins both charges. On the other hand, depending on the sign of the atom, the sense can be of attraction (+/-) or of repulsion (+ / +, - / -).
Based on all these premises, a series of conclusions that are as clear as they are fascinating can be drawn: charges with the same sign experience an electric force that tends to separate them, charges with a different sign experience a force that tends to unite them and the closer the charged atoms are, the greater the modulus of the electric force of attraction or repulsion.
- You may be interested in: "Action potential: what is it and what are its phases?"
Limitations of Coulomb's law
Despite being a revolution in its day and continuing in force today, it should be noted that Coulomb's law also reports certain limitations. Among them, we find the following:
- The loads must present a symmetric spherical distribution.
- Loads must not overlap.
- The charges must be stationary with respect to each other.
- For very small distances (on the order of the size of atoms), the electrostatic forces are outweighed by others, such as strong or weak nuclear forces.
The biological utility of electrostatic pressure
The fact that there are positive and negative atoms is not only useful at the level of knowledge. For example, ions are essential in the functioning of biological systems, both muscular and neurological, and of all organic tasks. Let's look at a concrete case in which the electric potential is transformed into tangible acts.
When a muscle is at rest, the attractive forces between the actin and myosin that compose it are inhibited. If we develop the desire to perform a specific movement (such as to frown), we emit an action potential (a wave of electrical discharge) that travels through neuronal synapses to the membrane of the motor neuron (motor neuron) related to that muscle we want contract.
These electrical potentials cause the motor neuron to release a chemical message to muscle tissue, transforming this order into the release of acetylcholine that binds to the receptors of the membrane of the muscle. This change in membrane potential on the muscle surface allows the opening of ion-dependent channels within cells., which translates into a massive influx of calcium ions (Ca 2+) after a series of steps, changing the conformation of muscle actin and myosin and allowing contraction.
Resume
As you can see, electrostatic pressures or electrical forces are everywhere. Electricity not only modulates the behavior of a light bulb or a battery, but, in the broadest sense of the word, allows us to transmit nerve signals to all parts of our body and respond to environmental stimuli in the most effective way possible.
In the end, everything is a game of charges: atoms or molecules with the same charge repel each other, while those with charges different are attracted, ideally with a force in a linear direction that will be greater the closer the two are bodies. With these premises, we can describe bonds such as ionic and covalent or the potential of cell membranes itself, therefore, life itself and the atomic organization of living beings. Without a doubt, without electricity we are nothing.

