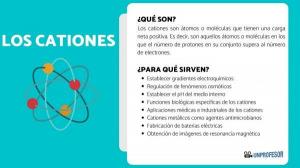
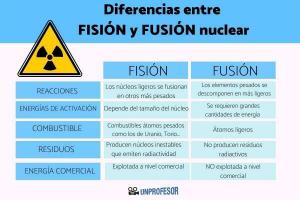
Exceptions to the Octet Rule
Welcome to UnProfesor, in today's video we will see some exceptions to the octet rule.
We have basically 3 situations:
- Odd number of electrons: if we have an odd number of electrons, it is difficult for all atoms to adopt the noble gas structure (with 2 or 8 electrons) and therefore they will not comply with the octet rule.
- Incomplete octet: some of the atoms will not meet the octet. This happens to us when we have compounds formed by Boron (B), Beryllium (Be) and Aluminum (Al) mainly.
- Extended layers: the central atom can exceed the octet, and this is because we have atoms that correspond to the third period and have electrons located in D-type orbitals, which means that they can accept more electrons than would correspond to them due to their structure of Lewis.
If you have any questions or comments about exceptions to the octet rule, you can do it through our website. And if you want to practice more, you will find below this video, some printable exercises with solutions for you to do.