5 DIFFERENCES between FISSION and nuclear FUSION

Classical chemistry holds that atom it is the smallest and indivisible unit of matter. Chemical reactions are those in which the atoms that make up the molecules recombine to give rise to new molecules as a result of the interaction of those initially present. However, atoms can establish interactions involving the particles that make up their nuclei. They are the so-called nuclear reactions.
In this lesson from a TEACHER we will discover what are the types of nuclear reactions and what are the differences between fission and nuclear fusion.
Both fission and nuclear fusion are nuclear reactions. These are processes in which atomic nuclei or atomic nuclei and subatomic particles interact with each other. The simplest nuclear reaction, and the first to be discovered, is the radioactivity, which consists of the spontaneous decomposition of an unstable atomic nucleus into a simpler one with greater stability and lower energy. This decomposition reaction releases energy in the form of radiation.
The remaining types of nuclear reactions are, generally, two nuclei or particles that react to give rise to the products of the reaction. For a nuclear reaction to occur, a activation energy. Nuclear reactions release energy in the form of kinetic energy (energy associated with motion) from atoms product of the reaction and, sometimes, to the emission of gamma rays (electromagnetic radiation of high energy).
Definition of nuclear fusion
As the name implies, nuclear fusion is the type of reaction in which two light cores fuse to form a heavier core, but with a mass slightly less than the sum of the masses of the two nuclei from which it was formed. This difference between the final mass and the initial mass is given off in the form of energy according to the reaction: E = m · c2.
Definition of nuclear fission
Nuclear fission is the opposite reaction to fusion. It is a reaction in which a heavy core, being bombarded by particles breaks down to give rise to lighter cores, also generating other products of the reaction such as subatomic particles and gamma rays.

Image: Mate fitness
Now that you know the meaning, let's dive right in to discover the differences between fission and nuclear fusion. There are five main ones and here we summarize them.
1.- These are opposite reactions
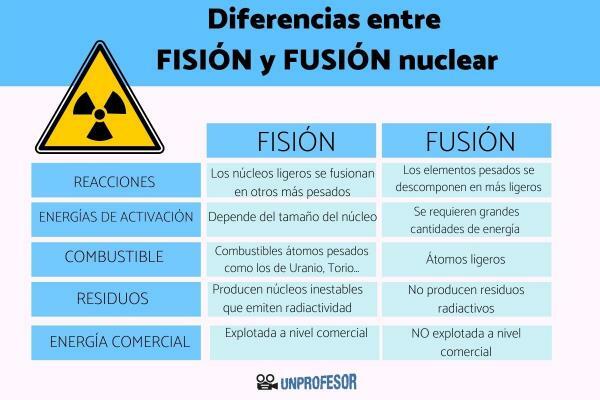
As we have commented in the previous section, fusion and fission they are two opposite nuclear reactions. Since in fusion light nuclei fuse into heavier ones and in fission nuclei of heavy elements decompose into lighter ones.
2.- Activation energies
- Fission: In the case of fission reactions, the activation energy depends on the size of the nucleus, in the case of heavy nuclei, the reaction occurs spontaneously. In the case of lighter nuclei, the reaction must be induced by bombarding the nuclei with low-energy particles. Therefore, in the case of nuclear fission the amount of energy required to start the reaction is very little or nonexistent.
- Fusion: In the case of nuclear fusion, require large amounts of energy to activate the reaction. To start the nuclear fusion reaction, it is necessary to raise the temperature of the fuel to 100 million ºC, so that the fuel atoms move to the plasma state (a state in which the electrons move freely independently of the nuclei atomic). This type of condition is the one that occurs inside stars, where nuclear fusion reactions take place.
3.- Fuel abundance
- Fission: These nuclear reactions require heavy atoms such as Uranium, Thorium or Plutonium as fuel. Heavy elements are the least abundant in the universe and they are in small proportions in the earth's crust. Furthermore, the radioactive isotopes of these heavy elements are found in nature mixed with other non-radioactive isotopes or as part of minerals.
- Fusion: The light atoms that participate in nuclear fusion reactions are the most abundant in the universe, where hydrogen (the lightest element) is the majority, representing 92% of the total. Although hydrogen is relatively scarce on Earth, it can be obtained from renewable sources such as cellulose biomass or from water. For this reason, hydrogen is considered to be a inexhaustible fuel.
4.- Residues resulting from the reaction
- Fission: Nuclear fission reactions produce unstable nuclei that emit radioactivity for very long periods of time, because they have half-lives (the period necessary to reduce radioactive emissions by half) that can be longer than 30 years. The radioactive waste production In nuclear fission reactions, they are a danger to human health and the environment, so they must be managed and stored properly.
- Fusion: Nuclear fusion reactions do not produce radioactive waste, which is why it is considered clean energy, since they are reactions that they do not produce polluting residues. In the case of the proton-proton fusion reaction, one of the most common fusion reactions, the product obtained is a noble gas, Helium. Helium is an element, very little reactive and that does not pose any danger to human health and the environment.
5.- Obtaining energy at a commercial level
- Fission: Currently, it is the only type of nuclear reaction that has the necessary technique to be exploited commercially. All nuclear power plants use nuclear fission reactions.
- Fusion: Today, we do not yet have the necessary technology to obtain electrical energy through nuclear fusion. The main technical difficulty is the high temperature necessary to start the reaction, since We do not have any material that can withstand these temperatures and where it is possible to confine the reaction.