Formulation of nonmetal nonmetal and metal nonmetal binary compounds
In this video I will explain the formulation of the metal - nonmetal, and nonmetal - nonmetal binary compounds. . It must be very clear that they are oxides and metals and non-metals.
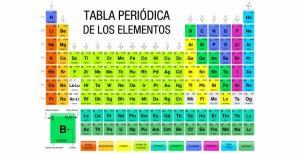
The first thing to define is that oxides will always have a component that has the Oxygen. When formulating, you have to know which of the two elements is ahead and we will know that depending on the element more electro positive and what is the less electro positive.
Metal - Non-metal
Metal is always much more electropositive than non-metal. Therefore, the one that will go first will be the metal, and then the non-metal.
Later we will have to know the oxidation state of each one. I exchange the valences.
Non-metal - Non-metal
It is more complicated than in the previous combination. We need to know at all times which is the most electronegative and which is the most electropositive. We will have to take into account the classification that you can see in the video.
In the video you will be able to see the formulation of the metal - nonmetal, and nonmetal - nonmetal binary compounds
. But if you are not completely clear about it, you can continue practicing with problems of this type by doing the printable exercises with their solutions that I have left you on the web. Good luck in your studies!If you want to read more articles similar to Formulation of nonmetal nonmetal and metal nonmetal binary compounds, we recommend that you enter our category of The atom.