Evolution of the PERIODIC TABLE: from its creation to today
The periodic table is one of the most iconic icons of the science. Although 2019 was the 150th anniversary of its creation, it is by no means a finished document. In this lesson from a TEACHER we will see what the evolution of the periodic table from its creation to the present day and what advances in the knowledge of atoms and their properties have made it possible.
Index
- What is the periodic table?
- First periodic table: the origin
- History of the periodic table and evolution
What is the periodic table?
The periodic table The periodic system of elements is the scientific document that concentrates more information in less space and constitutes one of the most powerful icons of science. It contains a good part of the knowledge we have about chemistry. There is no similar document in any other scientific discipline.
The periodic table of the elements is a classification system of chemical elements It started more than 200 years ago. This classification system has been growing and changing over time, as science progressed and new ones were discovered
chemical elements. However, the various modifications have been incorporated keeping its fundamental structure intact.The periodic table is so named because expresses graphically the way they repeat at regular intervals certain chemical properties. It is a type of two-dimensional representation or, in its more modern representations, three-dimensional.
In the classical periodic table (two-dimensional) the chemical elements are arranged in groups or families and are represented in the current periodic table in vertical columns. The ordered arrangement of these groups in columns gives rise to a series of rows, which are called periods, where the elements are ordered according to their atomic weight. The periodic table consists of seven periods that vary in length.

First periodic table: the origin.
Before Mendeleev, other scientists had developed classification systems for chemical elements. But, unlike the periodic table of the elements, they were mere lists of the known elements; while the periodic table has the particularity that it is a two-dimensional classification system (rows and columns) or three dimensions, in its most modern versions, where the chemical elements are arranged in successive layers.
For this reason, historians date the birth of the modern periodic table to February 17, 1869, when Dimitri Ivanovich Mendeleev finish the first periodic table of the many that he made. This table consisted of 63 elements ordered in families and left empty spaces for elements not yet discovered, but from which he had deduced their atomic weight (as in the case of Gallium, Germanium, and Scandium)
The key data for the discovery of the periodic table was the previous knowledge of the atomic weight of each element.
What is atomic weight and atomic number?
This number represented the weight of the atom and it was the only measurable value of the atoms. But it was not in any case of direct measurements (there are no measuring devices that allow weighing isolated atoms) but rather they were a system that established a standard in which an arbitrary value of 1 was given to the hydrogen atom and the value of the atomic weight of the remaining elements was calculated in relation to this Pattern.
The first calculations of the atomic number of the elements were carried out by the English chemist John dalton, and generated a great scientific debate during the first half of the 19th century. However, in the second half of the 19th century, there was already a remarkable consensus on the system for calculating the atomic weights of elements. The atomic weight became from Mendeleev onwards, a key criterion for the correct ordering of the elements within the periodic table.
When Mendeleev ordered the known elements according to their increasing atomic weight, he observed the appearance of recurring properties that allowed the elements to be grouped into groups or families of elements similar to each other. However, in some cases, the ordering of the elements according to their atomic weight did not respond to the similarities between elements that were observed and Mendeleev changed the position of 17 elements in the arrangement of the periodic table despite their atomic weights, in order to be able to group them with those elements with which they presented analogies.
These changes showed that some of the accepted atomic weights were not correct and were recalculated. Despite the corrections in the atomic weights, there were still elements that had to be placed in positions other than those indicated by their atomic weights.
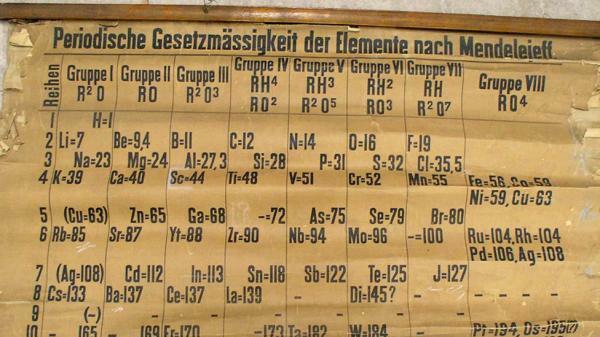
Image: BBC.com
History of the periodic table and evolution.
Despite Mendeleev's undoubted contribution, the periodic table of elements is not the result of the work of a single researcher. In addition to Mendeleev, during the second half of the nineteenth century and throughout the twentieth century many Chemists continued to investigate the best way to organize information on chemical elements known. Even more so considering that during this time the discovery of new chemical elements or simple substances, thanks to spectrometry (which studies the interactions between atoms and electromagnetic radiation).
The classification of elements in the periodic table was initially based on the incipient calculations of the atomic weights of the elements and revealed analogies that allowed the elements to be grouped into analogous families. Even so, the reason for the appearance of these periodic properties could not be explained. It was throughout the 20th century, with the discovery of electronic structure, when the reason for this periodicity in the properties of the elements was understood.
The atomic number as a sort order
At the beginning of the 20th century Glover and Rutherford, observed that the charged particles in the nucleus accounted for about half the atomic weight. This value corresponds to the concept of atomic number which is defined as the number of protons in the atomic nucleus and which coincides with the number of electrons in a neutral atom. This new value justified the changes of position of certain elements that had been carried out until then. For example the change of position between Tellurium and Iodine.
In 1913, Henry Moseley he confirmed the ordering of the table as a function of atomic number by X-ray spectrometry. The ordering according to the atomic number is still in force today.
At the same time, during the 20th century, new chemical elements continued to be discovered thanks to quantum mechanics and the development of the technique of bombardment of atoms by particles, from the second half of the century. With this new technique it was possible to create artificial elements that are not present in nature.
Although he had made progress in the correct arrangement of the elements within the periodic table, he still the reason for the recurring occurrence of certain properties (the properties periodic). The development of the quantum mechanics (branch of physics that studies the behavior of light and atoms on a microscopic scale) from 1920 was decisive to explain the reason for these properties.
Electronic configuration as an explanation of periodic properties
During the first half of the 20th century, physicists Niels Bohr Y Wolfgang Pauli they proposed an atomic model in which electrons can only occupy certain orbits and where the electrons are arranged forming layers of different energy levels. The way in which the electrons are distributed in the orbitals in the different shells or energy levels is known as electronic configuration.
The discovery of the arrangement of electrons in electron configurations was fundamental to understanding the periodicity of properties. periodic, since it was observed that these properties were closely related to the outermost electronic configuration of the atoms (layer of Valencia).
The order in which electrons fill the atomic orbitals was established in 1930 by physicist Erwin Madelung who established a numerical rule for the order of filling. This rule is known as Madelung rule and it is an empirical rule that could not be explained by means of quantum mechanics.
The filling sequence is simple for the first three rows of the periodic table, but in the fourth row, where the transition elements are located, the order of filling undergoes a series of alterations. There are a total of 20 anomalous items that do not follow this rule.
Evolution of the table continues today
In 2006, the theoretical chemist Eugen Schawrz He managed to explain the anomalies of Madelung's rule taking into account that atoms can have different electronic configurations depending on the energy level. Calculating the averages, the electron configurations of most elements do comply with Madelung's rule.
The periodic table continues to be a matter of debate in the 21st century, although the arrangement or electronic configuration of the elements, it is still valid to explain this ordering and the anomalies observed in the electronic configurations by means of a theory.
If you want to read more articles similar to Evolution of the periodic table - summary, we recommend that you enter our category of The atom.
Bibliography
Various authors. (2019)Special: the periodic table. Research and science. Barcelona: Scientific Press S.A.

