Nomenclature of metal and non-metal oxides
In this video I will explain the how to name metal and nonmetal oxides. It must be clear that they are oxides and metals and non-metals.
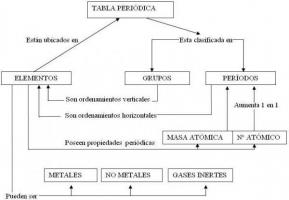
The first thing to define is that oxides will always have a component that has the Oxygen. When formulating, you have to know which of the two elements is ahead and we will know that depending on the element more electro positive and what is the less electro positive.
There are two naming systems: IUPAC (systematic) and Stock. Stock is the most common.
We always put the most electropositive element, and then the most electronegative. We reverse the order when naming them. First we name the most electronegative, and then the most electropositive.
We indicate the proportion in each compound by prefixes: mono, tri, tetra, etc.
In the video you will be able to see how to name metal and nonmetal oxides. But if you are not completely clear about it, you can continue practicing with problems of this type by doing the printable exercises with their solutions that I have left you on the web. Good luck in your studies!