CLASSIFICATION of METALS from the periodic table

The metals are the more abundant elements on the periodic table. They are characterized by: being good conductors of heat and electricity, being solid at room temperature (with the with the exception of mercury which is liquid) and have the ability to reflect light, so they have a characteristic.
But this great set of elements is presented ordered within the periodic table in different groups or families that reflect their similarities and differences. In this lesson from a TEACHER we will see what the classification of metals and what are the characteristics and properties of the different groups defined in this classification.
As we have already mentioned, metals are the majority elements of the periodic table. They are distributed in two great guys that include, in each case, various subtypes that are grouped into families of the different blocks of the periodic table.
Below we present a brief outline of this classification, which we will develop in more detail in the following sections.
- 1. Representative metals: s block of the periodic table.
- 1.1. Family of alkali metals
- 1.2. Family of alkaline earth metals
- 2. Transition metals: block d of the periodic table.
- 3. Internal transition metals: block f of the periodic table.
- 3.1. Lanthanides: elements of the 6th period of the table.
- 3.2. Actinides: elements of the 7 period of the table.
- 4. Post-transitional metals: p block of the periodic table.
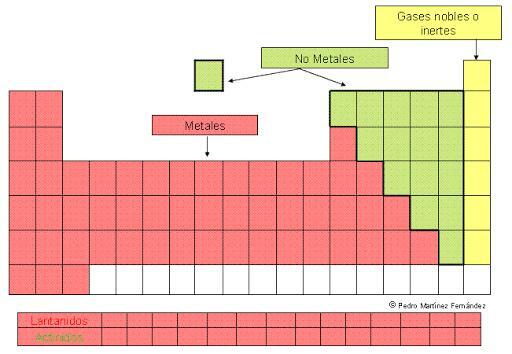
Image: Educamix
The representative elements or main elements are those elements that are more abundant in nature. Among the metallic elements, representative elements are the metals of the block s, that is to say the alkaline elements (family 1 of the periodic table) and the alkaline earth elements (family 2 of the periodic table).
In these two groups we find very reactive elements, with a strong tendency to oxidize (lose their electrons from the valence shell) and therefore, they are powerful reducers of other elements. In nature they are found in the form of ionic salts very soluble in water, oxides or hydroxides (strong bases).
Alkali metals (group 1 of the periodic table)
- The alkali metals represent 5% of the earth's crust. Sodium (Na) and Potassium (K) they are the most abundant.
- They are shiny elements silvery in appearance, low density, soft metals, and highly reactive. Due to their high reactivity, they are not found in their pure state in nature. The boiling or evaporation points of alkali metals are relatively low and they are good conductors of heat and electricity.
- From the point of view of their electronic configuration, they are elements that present a single electron occupying the s orbital of its valence shell. They have a combining power of 1 (valence) and oxidation number +1. They show a great tendency to lose the electron from the outermost shell to form cations.
- As bioelements, alkali metals develop a important role in living organisms, especially sodium and potassium, which play a fundamental role in nerve transmission and, in the case of potassium, in regulating enzyme activity.
- These metals have multiple uses in industry. For example, Lithium (Li) is used to produce high-strength aluminum alloys, in ceramic production, or as battery components. It also has medical uses since it constitutes a component of the nervous system and its deficiency causes psychiatric diseases.
Note: Group 1 of the periodic table also includes Hydrogen, which is not a metal.
Alkaline earth metals (group 2 of the periodic table):
- The alkaline earth metals represent 4% of the composition of the earth's crust. They are especially abundant calcium (Ca) and Magnesium (Mg).
- Like the alkali metals, these metals they are very reactive therefore, they are not found in free form in nature.
- Although they have similar physicochemical characteristics to alkali metals, they tend to be harder and less reactive than alkali metals. They have low densities and hardnesses and melting points higher than those of alkali metals.
- From the point of view of electron configuration, they are characterized by present the s orbital of the valence shell filled (that is, occupied by a pair of electrons). Therefore, they have a combining power of 2 (valence) and an oxidation number of +2. They easily react with halogens (group 17 of the periodic table), to form ionic salts.
- Your role as constituents of living organisms it is especially important in the case of calcium (Ca) and magnesium (Mg). Magnesium and calcium ions are the most abundant ions in seawater together with the chloride ion (Cl-).
- 99% of the calcium in our body is found in the skeleton, but in its ionic form has a fundamental role in nerve transmission, neuromuscular function and regulation enzymatic.
- Magnesium, in its ionic form, performs important biological functions in living organisms, including the most prominent, its fundamental role in the photosynthesis of plants as a component of the chlorophyll.
- The industrial uses of alkaline earth metals are diverse. The most relevant are the use of calcium as a component of cement, the use of magnesium for the elaboration of fires artificial, as a coating of iron structures to prevent their oxidation or as a component of alloys and steels light.
Image: Google Sites
Within the classification of metals we have to talk about transition metals or metals of the block d, are the most abundant group of metals and are grouped in a total of 10 groups or families of the periodic table.
- Most transition metals have characteristics similar to representative metals: they are good conductors of heat and electricity and reflect light.
- They show great variability in terms of hardness and boiling and melting points but, in general, they are harder and have higher melting and boiling points than alkali metals and alkaline earths.
- From the chemical point of view they are characterized by: having multiple coordination numbers (valences) or oxidation states, they are usually good catalysts (ability to increase or decrease the rate of chemical reactions) and form compounds with color and have the ability to form coordination complexes (chemical compounds with a metal ion in the center, attached to a series of ligands arranged at their around). For this reason, transition metals form cations of different charges.
- Density is highly variable in this block of elements, from strontium with a low density to osmium (Os), which is the element with the highest density in the periodic table.
- If we look at the electronic configuration of transition metals, they are characterized by presenting partially filled d orbitals. The filling of the orbitals in this block of the periodic table presents a series of irregularities, which are reflected in the multiple oxidation numbers acquired by the metals in this block of the table periodic.
Iron (Fe) and titanium (Ti): more abundant transition metals
- Iron is the most abundant and represents about 5% of the weight of the earth's crust. It is rare to find it in its elemental form in nature, where it is normally found forming oxides and carbonates.
- Pure iron has few uses, but its alloys with other substances have multiple uses. Useful forms of iron alloys are wrought iron (it is an iron alloy characterized by its low carbon content and high iron content. It has the property that it can be red-hot molded and hardens on rapid cooling), cast iron (which is also known as the name of gray cast iron or cast iron, it is an alloy of iron, silicon and carbon that contains small amounts of manganese, phosphorus and sulfur; in which carbon is in the form of graphite) and steel (a purified alloy of iron and carbon).
- Other transition metals widely used in industry include copper and silver. In addition, many transition metals are used in industry as catalysts for chemical reactions.
- At the biological level, iron in its ionic form has a fundamental role in the oxygen transport, since it is part of the active center of hemoglobin and myoglobin.
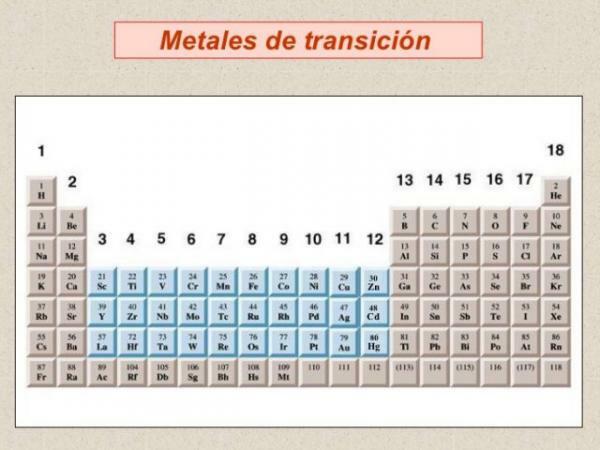
Image: 100cia site
The internal transition metals or metals of the block fThey are also called rare earths. They are grouped into two families of elements: lanthanides and the actinides. They are those metallic elements in which electrons are found occupying the f orbitals. The elements of the group of lanthanides have partially occupied the f orbitals of level 4 and the actinides those of level 5.
Lanthanides or lanthanoids
- They are the 15 elements of internal transition that are part of the period 6 of the periodic table of the elements.
- This group of elements has common characteristic properties. Is about soft and silver-shining metals, Its conductivity of heat and electricity is relatively low compared to other metals. They are metals of lower density than transition metals.
- In nature, they are found in low proportions, forming part of many minerals. Lanthanides have a high capacity for magnetization or magnetization and are also characterized by the luminescence of their cations.
- Lanthanides have multiple uses in industry in the production of strong permanent magnets, rechargeable batteries and the manufacture of superconducting materials. They have multiple applications in optics (manufacture of fluorescent tubes and lamps, liquid crystal displays and lasers). They are also used as catalysts for chemical reactions or as pigments.
Actinides or actinoids
- They are the 15 elements that make up the period 7 of the periodic table.
- Many of them have been artificially synthesized, but they are also found in nature in very small proportions.
- They show a behavior similar to that of the transition metals (block d) and different from that of the lanthanides. As in the case of many metals, they have a characteristic silvery sheen.
- As a group, their importance lies in the fact that they are all radioactive elements. That is, these are elements whose nuclei are unstable disintegrate, releasing energy (nuclear energy) and give rise to other chemical elements with a more stable nucleus. All isotopes of the elements in this group are radioactive and have a short half-life. The most abundant actinides in nature are uranium (U) and thorium (Th).
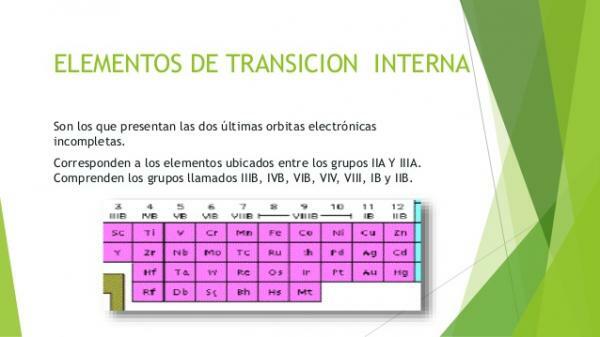
Image: Slideshare
