Nomenclature of nonmetal nonmetal and nonmetal metal binary compounds
In this video I will explain how we can name the binary compounds formed by a metal and a nonmetal, or by two nonmetals.
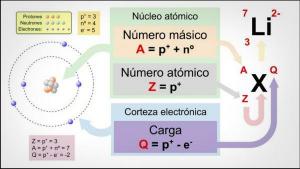
In the formulation the most electropositive element is placed, followed by the most electronegative. At the time of naming them we reverse the order, first we will name the element most electronegative and then the plus element electronegative.
There are two naming systems: the IUPAC (systematic) and Stock.
IUPAC:
M- NM
(Mono / oli / tri… + -uro) + de + (mono / di / tri… + m / nm)
NaCl = monosodium monocuride
NM- NM
Cl2O3 dichloro trioxide
Stock:
M- NM
M + -uro) + de + m / nm (valence)
NaCl = Sodium chloride
NM- NM
Cl2O3 chlorine (III) oxide
In the video you will be able to see how we can name the binary compounds formed by a metal and a nonmetal, or by two nonmetals.. But if you are not completely clear about it, you can continue practicing with problems of this type by doing the printable exercises with their solutions that I have left you on the web. Good luck in your studies!
If you want to read more articles similar to
Nomenclature of nonmetal nonmetal and nonmetal metal binary compounds, we recommend that you enter our category of The atom.