MOLECULAR geometry: definition and examples
The three-dimensional shape in which the atoms that make up a molecule are arranged is known by the name of molecular geometry or molecular structure.
It is possible to deduce the geometry of these molecules from a theoretical model: the repulsion model of the pairs of electrons in the valence shell (RPECV). This model is especially useful for representing the geometry of molecules made up of small atoms and linked together by covalent bonds (electron sharing).
In this lesson from a TEACHER we will discover the definition of molecular geometry and examples So that, in this way, you learn what the RPECV model consists of, how the geometry of molecules can be deduced by this method and some examples.
Index
- Definition of molecular geometry
- Examples of molecular geometry
- Know the Lewis structure of the molecule
- Electron pair repulsion model of the valence shell (RPECV)
Definition of molecular geometry.
Molecular geometry or molecular structure is the way atoms found a molecule are arranged in space.
This three-dimensional structure (molecular geometry) comes defined by a series of forces that hold the atoms together in a specific arrangement. Among the forces that determine molecular geometry the most important are the links that the atoms establish each other for form the molecule.
The geometry of the molecules is very important because it determines what are the physicochemical characteristics of matter. For example: H2O molecules have an angular geometry that is given by the bonds that form it. Adopting this angled geometry makes the water molecule an electric dipole and has exceptional properties. Thanks to its geometry, water is liquid at room temperature, it is capable of dissolving many substances, etc.
Obviously, given the size of the molecules, their geometry cannot be observed directly and must be deduced by indirect methods. Furthermore, it is necessary to represent these geometries by means of theoretical models.
It is these theoretical models that allow us to determine what the geometry of a molecule is like from its molecular formula.
Examples of molecular geometry.
As we have seen in the previous section, the atoms that make up a molecule can acquire different spatial arrangements (geometries). In this section we will see some examples of molecular geometry.
Two-dimensional geometries
In some cases, the molecules acquire flat or two-dimensional geometries, that is, they are structures that only have two dimensions and occupy a surface (they do not have volume).
Linear geometry
It is the simplest geometry, it is about molecules whose atoms are joined to form a straight line. All molecules made up of two atoms are linear, but this geometry also occurs in molecules made up of three atoms.
Examples of linear molecules:
Formed by two atoms: all diatomic gases such as O2, H2.
Made up of three atoms: CO2 (Carbon dioxide).
Angular geometry
They are molecules made up of three atoms that come together at an angle. The amplitude of the angle formed can be different, depending on the type of atoms that form it. The amplitudes of the angles formed by the angular molecules have values between 90º and 120º.
Examples: H2O, SO2 (sulfur dioxide), SnCl2 (tin dichloride)
Triangular geometry
They are molecules made up of four atoms, with one atom located in the center of an imaginary triangle and the other three remaining atoms located in each of the vertices of this triangle.
Examples: SO3 (sulfur trioxide), NO3- (nitrate ion)
Square geometry
Molecules with this geometry have 5 atoms. One is located in the center of a square and the other 4 in each of the vertices of the figure.
Examples: XeF4 (Xenon trifluoride)
Three-dimensional geometries
They have three dimensions, that is, they have volume. The geometries of 3D molecules is very diverse, here we will see just a few examples.
Tetrahedral geometry
This geometry is the one presented by some molecules formed by five atoms, in it an atom is located in the center of an imaginary cube and the four remaining atoms are located at the vertices of the cube (tetrahedron).
Example: CH4 (methane), MnO4-(permanganate ion)
Trigonal pyramidal geometry
They are molecules with four atoms arranged at the four vertices of a pyramid with a triangular base.
Example: NH3 (ammonia), PH3 (phosphine)
Quadrangular pyramidal geometry
In this case the number of atoms that make up the molecule is six and five of them are arranged in the vertices of a pyramid with a square base, while the sixth occupies the center of the square of the base.
Example: ClF5 (chlorine pentafluoride)
Know the Lewis structure of the molecule.
Before you can use the RPECV method it is necessary to know what it is Lewis structure of the molecule and for this you must first know what the electronic configurationfrom the Valencia layer of the different atoms that make up the molecule.
Therefore, before being able to determine the geometry of a molecule it is necessary to carry out some previous steps:
- TO. Get the electron configurations of the different atoms that make up the molecule.
- B. Determine the number of valence shell electrons of each of the atoms. The electrons in the valence shell are the electrons that the atom can use to form bonds.
- C. Deduce the Lewis structure taking into account how many electrons each atom has in its valence shell.
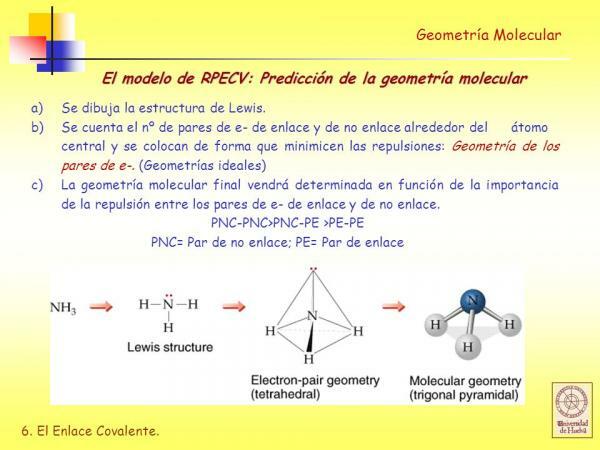
Image: Slideplayer
Electron pair repulsion model of the valence shell (RPECV)
In the Lewis structures each of the bonded atoms, must meet the octet rule. When an atom fulfills the octet rule, it is surrounded by four pairs of electrons that can be electrons that are part of a bond (bonding electron pairs) or electron pairs that do not participate in bond formation (non-bonding electron pairs) binding).
As we will see, once the Lewis structure of a molecule is determined, deduce its geometry using the repulsion model of the valence shell electron pairs is very easy.
According to this representation model, the ligands (X) and the non-bonding electron pairs (E) are arranged around the central atom (A), so that the distance between them is the maximum. The sum of ligands and nonbonding electron pairs (X + E) determines the type of geometry of the molecule.
X + E = 2
Linear Geometry
AX2: Molecule formed by two ligand atoms attached to a central atom
Example: beryllium hydride (BeH2).
X + E = 3
Triangular plane geometry (equilateral triangle)
AX3: Molecule made up of three atoms attached to a central atom
Examples: Some chlorides such as boron or aluminum (BCl3, AlCl3)
Angular geometry (120º angle)
AX2E: Molecule with a central atom attached to two ligands and a nonbonding electron pair.
Examples: Tin (II) Chloride (Sn2Cl)
X + E = 4
Tetrahedral geometry
AX4: Molecules with a central atom with four ligands arranged in bonds so that the ligands are they have at the vertices of the opposite diagonals a cube whose center is the central atom itself.
Examples: Molecules such as methane (CH4), silicon chloride (SiCl4) or carbon tetrachloride (CCl4) present this geometry.
Trigonal Pyramid Geometry
AX3E: Molecules with 3 ligands and 1 lone electron pair in which the atoms of the three ligands are arranged to form the base of a pyramid with a triangular base in which the central atom is in the upper vertex of said pyramid
Examples: one of the molecules that has this geometry is ammonia (NH3).
Angular geometry (109º angle)
AX2E2: The two ligands and the central atom are arranged forming an angle of 109º
Examples: Water (H2O) is one of the molecules that have this geometry.
Glinear eometry
AX3: Since there is only one ligand attached to the central atom, the geometry is linear.
Example: Hydrogen fluoride or hydrofluoric acid (HF).
X + E = 5
Trigonal bipyramidal geometry
AX5: The molecule has the geometry of two opposing pyramids, with a triangular base common to both. The central atom is arranged in the center and the ligands are located at the vertices.
Example: Phosphorous pentachloride (PCl5)
Dyshenoidal geometry
AX4E: In this type of geometry, the atoms acquire an arrangement that resembles the structure of a seesaw swing.
Example: Tetra sulfur fluoride (SF4).
T geometry
AX3E2: Molecules are shaped like the letter T, with the ligands at the ends of the letter and the central atom at the point where the two lines that form it meet.
Example: Chlorine trifluoride (ClF3)
Linear geometry
AX2E3: In this case, the three atoms of the molecule are arranged in line with the central atom in an intermediate position.
Example: Xenon Difluoride (F2Xe)
X + E = 6
Octahedral geometry
AX6: This type of molecule has a structure that resembles an octahedron in which the central atom would occupy the center of the geometric figure and the six ligands each of its vertices.
Example: Sulfur hexafluoride (SF6)
Square base pyramid
AX5E: In this case, the atoms form a figure in which the central atom occupies the center of the base and the ligands the five vertices of the figure.
Example: Bromine Pentafluoride (BrF5)
Plane square geometry
AX4E2: The atoms acquire a square-shaped arrangement, in which the central atom occupies the center of the figure and the ligands each of its vertices.
Example: Xenon tetrafluoride ion (XeF4)

If you want to read more articles similar to Molecular Geometry: Definition and Examples, we recommend that you enter our category of The atom.
Bibliography
Alejandrina Gallego Picó, Rosa Mª Garcinuño Martínez, Mª José Morcillo Ortega, Miguel Ángel Vázquez Segura. (2018) Basic chemistry. Madrid: Uned
