Crookes tube experiment
During the 19th century, there were successive mysterious radiation discoveries such as radio waves, cathode rays, or X-rays. These discoveries consolidated the theory that nature emitted "emanations" that were capable of acting at a distance and that the senses could not perceive. In this lesson from a TEACHER we will discover how Crookes tube experiment it was one of the scientific investigations that contributed in a most decisive way to the discovery and understanding of cathode rays.
The invention of vacuum tube by Hienrich geissler was a fundamental step in the discovery of a series of mysterious radiations such as X-rays or cathode rays.
Vacuum pumps already existed in 1855, but the one invented by Geissler represented an important improvement, since managed to reduce the pressure inside a glass tube filled with gas to 0.01% of the pressure atmospheric. Geissler's vacuum tube was strong enough to reduce pressure down to very small values.
Over the next 50 years, Geissler's new vacuum pump enabled the emergence of fundamental inventions for the advancement of technology such as the
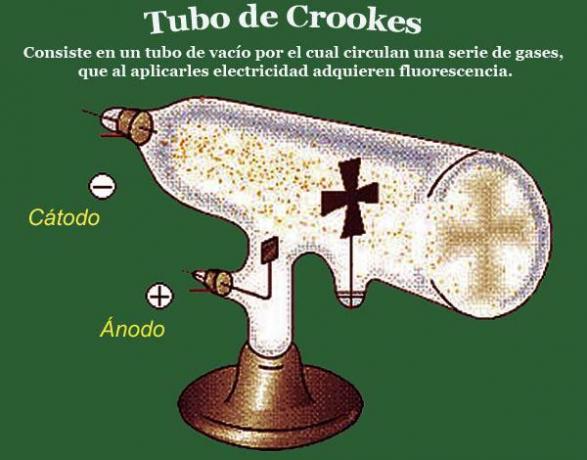
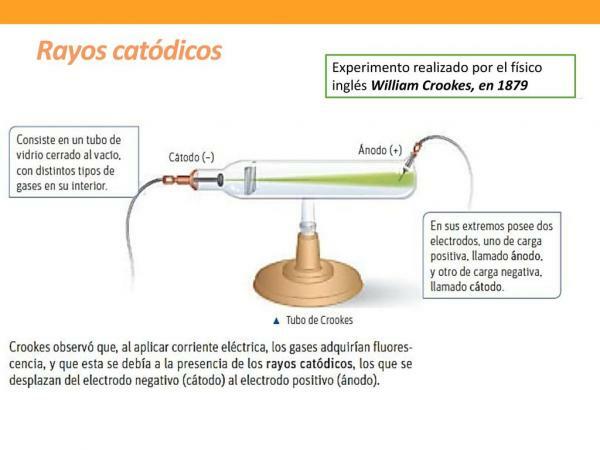
electric light bulb and opened new fields for research.Later, Julius Plücker incorporated electrodes to vacuum tube by Geissler. That is, it incorporated two metal plates (electrical conductor) connected to a current generator. The positively charged electrode is known as the Anode and the positively charged electrode is called the Cathode. Plücker observed that despite the vacuum the current continued to flow through the vacuum tube from the cathode to the anode, producing a pale green light.
Despite these discoveries, it would take two decades for an in-depth study of these light tubes to finally be carried out.
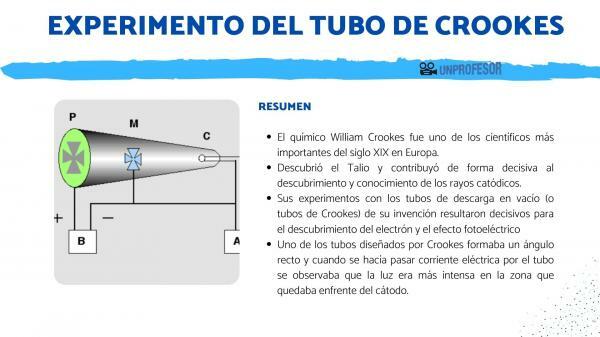
The chemist William Crookes He was one of the most important scientists of the 19th century in Europe, discovered Thallium, and contributed decisively to the discovery and knowledge of the cathode rays.
Crookes was a tireless inventor and noted for his great skill in the field of experimentation. His experiments with vacuum discharge tubes (or crookes tubes) of his invention were decisive for the discovery of the electron and the photoelectric effect.
In 1875 he had designed new vacuum tubes to study the nature of these luminous phenomena. William Crookes designed different vacuum discharge tubes. The best known are the three that were used in the experiments that allowed us to know what the characteristics of cathode rays were. Here we are going to offer a summary of what the Crookes tube experiment was like.
Angled Vacuum Discharge Tube Experiment
One of the tubes designed by Crookes formed a right angle and when electric current was passed through the tube it was observed that the light was more intense in the area that was in front of the cathode.
He also performed different experiments varying the pressure inside the tube and observed that the lower the pressure, the more intense the glow that was produced. He also tested plates of different metals as cathodes and found that the glow produced did not depend on the metal used as the electrode.
These results indicated that the light effect produced came from the cathode, and was independent of the type of metal used in the electrode. Due to these observations, the green light emitted by the cathode was named cathode rays.
Barrier Vacuum Discharge Tube Experiment
This is perhaps Crookes's experiment and tube best known, since it allowed to reach conclusions of great importance about the nature of cathode rays.
After what he observed in the first experiments with the angled tubes, Crookes set out to investigate penetrability of these rays, that is, to check if they were capable of crossing different barriers. For this, William Crookes designed vacuum tubes in which different barriers were installed, the best known of which is the zinc plate in the shape of a Maltese cross. The experiments carried out with this tube showed that the cathode rays were intercepted by the maltese cross shaped barrier; since a shadow with the shape of the cross appeared, in the middle of the luminescence, at the end of the tube.
To perform this experiment, Crookes designed a tube in which the cathode and the barrier (metallic malt cross) were located in a straight line, and he observed that the shadow that appeared at the end of the tube was also aligned with these two elements.
This experiment allowed him to reach the following conclusions:
- Cathode rays, like light, travel in straight lines and cast shadows.
- Cathode rays give off some kind of energy, since the end of the tube where they hit was heated.
Vacuum tube experiment subjected to a magnetic field
We finish this lesson of the Crookes tube experiment to talk about another very important one. William Crookes conducted multiple experiments to try to elucidate the nature of cathode rays. In one of them he subjected the vacuum tube to magnetic fields (moving a magnet in the vicinity of the vacuum tube) and observed that the cathode ray beam was deflected, which was not the case with light.
This experiment later made it possible to demonstrate that cathode rays were made up of negatively charged particles. Twenty years later J.Thomson managed to identify such particles as electrons.