Difference between glass and crystal
Glass is a material created by man from the combination and fusion of minerals at high temperatures, which results in a transparent, brittle, hard and moldable by-product.
The crystal is all solid present in nature formed from chemical processes that create a crystalline lattice, which is an ordered and symmetric structure of atoms and molecules.
The difference between glass and crystal is that glass is created with human intervention, while the crystal is a way in which the atoms and molecules of the bodies are grouped solid.
| Glass | Crystal | |
|---|---|---|
| Definition | Amorphous and moldable material created from the fusion of compounds at high temperatures. | Solid material formed from the crystallization process. |
| Source |
|
It is found in nature. |
| Types |
|
|
| Examples |
|
|
| How it is recycled |
Glass waste goes in the green bin. |
If you mean lead glass, commonly called "crystal," it cannot be recycled. Lead glass waste goes into the gray container. |
What is glass?

Glass is a solid and amorphous material, created from the fusion of compounds such as silicon oxide (SiO2), sodium carbonate (Na2CO3) and calcium carbonate (CaCO3), among others.
In nature, glass can be found:
- Like obsidian, a bright, strong and sharp rock obtained by the cooling of volcanic lava.
- How you tecticas, which are glasses from meteorites that hit the Earth and which are very rare due to their origin.
The use of glass in its natural form is not so common and has been reduced to the elaboration of ornamental objects, hence man-made glass is much more popular.
On the other hand, industrial glass is classified into several types, depending on its chemical composition.
Types of glass and their uses
There are 4 types of glass, according to the components that make it up.
Sodium-calcium glass

It is made up of calcium, sodium, and silica. It is very easy to melt and that is why it is the cheapest type of glass on the market.
For a long time it was recommended to avoid sodium-calcium glass for the manufacture of refractory containers for gastronomic use, because it did not tolerate changes in temperature and it used to break. However, this characteristic has been modified with the incorporation of a greater amount of silica, which gives it greater resistance.
An example of sodium-calcium glass can be found in automotive glass.
Lead glass

It is a glass that is manufactured by substituting lead for sodium and calcium. It is very easy to melt and expand, which means that it expands when melted. It also has a refractory and UV absorbing quality.
Lead glass is often called lead crystal, or simply crystal. However, this is only a commercial one, as glass is not a crystalline structure, therefore glass objects do not exist.
In everyday life, lead glass is present in glasses, glasses or dishes.
Borosilicate glass

It is composed of silica and boron oxide. It does not melt so easily, it has a great refractory capacity and its expansion capacity is limited, which is why it is used to manufacture laboratory and kitchen materials, as they can withstand high temperatures without expanding and without risk of breaking from shock thermal.
An example of application of borosilicate glass is refractory trays and containers.
Silica glass

It is the most difficult glass to melt, since very high temperatures (more than 1500 ° C) are required, as well as very sophisticated and expensive techniques to transform it into a final product.
Examples of silica glass can be seen on objects that require prolonged exposure to high temperatures, such as coatings for furnaces or laboratory tubes.
What is crystal?

The crystal is a solid, transparent structure, with an orderly, symmetrical molecular arrangement and regular geometry. It is abundant in nature, and is formed from crystallization, a process in which atoms or molecules form bonds to create an elemental unit called unit cell, a structure in the shape of a cube or parallelepiped.
In everyday life, the term "crystal" is used to refer to lead glass, used in the manufacture of objects such as glasses and goblets. This name is incorrect, since glass is a material with an asymmetric and disordered molecular structure, therefore, they are two different materials.
Unit cells are classified into various groups based on their characteristics. These groups are known as crystal systems.
Crystalline systems
According to the length of the sides of the unit cell, the arrangement of its axes and angles, crystals are classified into seven large crystal systems.
1. Cubic system
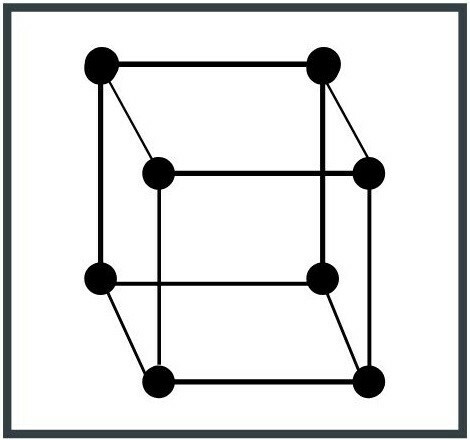
The unit cell is cube-shaped. It is the simplest system configuration and one of the most common in nature. Examples of cubic crystal systems are iron (Fe) and copper (Cu).
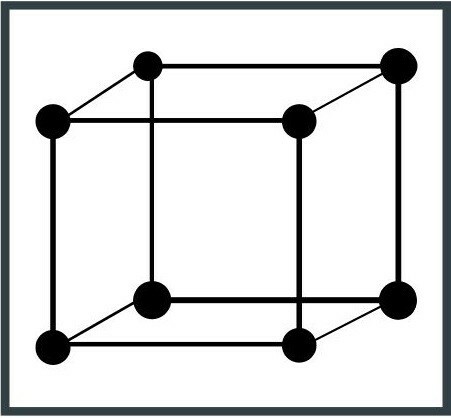
2. Tetragonal system
The unit cell is shaped like a parallelepiped, resulting in a figure with a three-axis base with 90 degree angles. An example of a tetragonal crystal system is manganese oxide (Mn4+OR2).
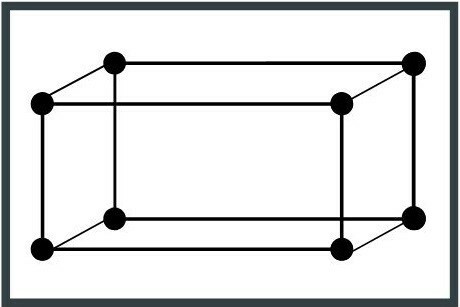
3. Orthorhombic system
The unit cell is shaped like an elongated cube with three right angles and three edges (segments that limit the faces of the cube) with different lengths. Topaz is a mineral that belongs to this crystal system.
4. Hexagonal system
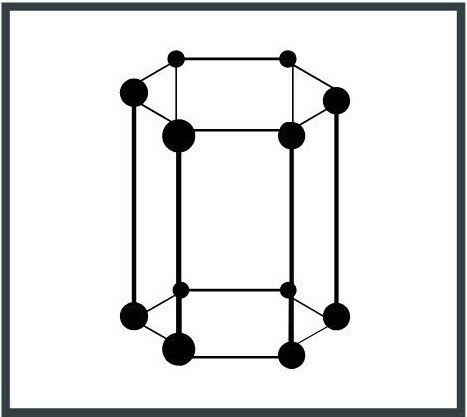
The unit cell has the base of a hexagon and the symmetry of a prism, with three axes of 120 °. An example of a hexagonal system is graphite, one of the forms in which carbon (C) occurs in nature.
5. Rhombohedral system

The unit cell has three right angles and three equal edges. Ruby is an example of a trigonal crystal system.
6. Monoclinic system
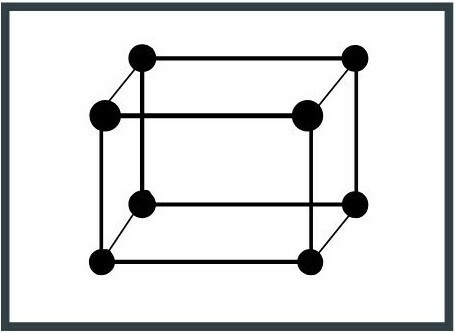
The unit cell has two 90 ° axes and its edges have different lengths. Mica is a mineral that has this configuration.
7. Triclinic system
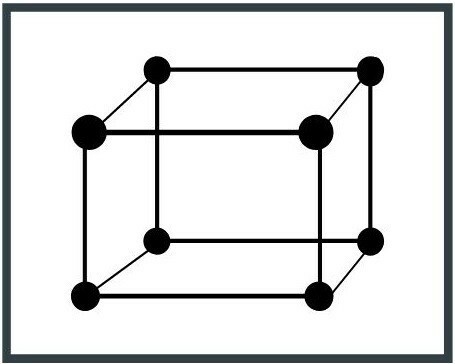
The unit cell has three unequal axes, as well as their lengths. An example of a triclinic system is albite (NaAlSi3OR8), a mineral from the group of silicates.
How is glass and crystal recycled?

Although they are treated as synonyms, glass and crystal are two different elements. Glass is a material resulting from the fusion of various compounds, such as silica, while glass is a molecular structure.
Glass recycling
Glass recycling consists of taking advantage of glass waste to convert it into raw material for the production of new products. To do so, you need to follow these steps:
- Classification: glass is classified according to its type (if it is sodium, lead, borosilicate, etc.)
- Separation: once classified, the glass is separated from any other material that has not been previously disposed of (small pieces of plastic, metal, etc.)
- Crushed and melted- Clean glass is crushed and melted together with other compounds such as sodium, limestone and silica to obtain the raw material that will be used in the elaboration of new products of glass.
The glasses suitable for recycling are those that come from bottles, containers and glasses and are deposited in the green containers. Laminated, broken, bulb or lamp glass cannot be recycled and goes in the gray container.
Glass recycling
When you talk about recycling glass, you are really talking about recycling lead glass. In this case, objects made from this material cannot be recycled and they go in the gray container.
Recycling the "crystal" or lead glass in green containers causes serious damage to the environment. Lead is a compound that is harmful to health, and if it is not properly separated in recycling centers, it ends up in smelting furnaces. There it will be mixed with other glass remains with which bottles or other objects will later be made.



