What are the VALENCES of NITROGEN

Nitrogen is a very important chemical element in our life, Both as for well and for worse. It is the main gas in the atmosphere, it is present in the soil and it is a very important macromolecule for most living beings. It is also part of compounds of great industrial importance such as ammonia, propellants or explosives.
What happens is that its valence and oxidation state is different depending on the compound. In this lesson from a TEACHER we will talk about what are the valencies of nitrogen. If you are interested in learning about this chemical element, you will like this article!
Index
- What is nitrogen and properties
- What are the valencies of nitrogen?
- Nomenclature of nitrogenous compounds
- Important Nitrogen Compounds
- Health Effects of Nitrogen
- Environmental effects of nitrogen
What is nitrogen and properties.
Nitrogen is a chemical element with symbol N. with an atomic number of 7, an atomic weight of 14.0067 and found in a gaseous state under normal conditions. Molecular nitrogen represents 78% of the volume in dry air and is therefore the main gas present in the atmosphere.
This high concentration of nitrogen in the atmosphere results from electrical action in the atmosphere, the fixation of atmospheric nitrogen by bacterial action, chemical action in industries and the release of nitrogen by the decomposition of organic matter or by the combustion. In its combined state forming compounds, nitrogen is found in different states.
It is an element of great importance for living beings, since It is part of all proteins both vegetable and animal, and many other organic compounds. Nitrogen forms strong bonds with other atoms such as nitrogen and others, due to its ability to form triple bondsTherefore, nitrogen compounds possess a large amount of energy.
Nitrogen consists of two isotopes:
- The N14 (very majority)
- N15 and various radioactive isotopes, which are produced during nuclear reactions.
It is an element of great interest in the chemical industry and in compounds used in agriculture. It is also used in incandescent lamp bulbs and when a relatively inert atmosphere is needed.
Nitrogen in its elemental form is slightly reactive at ordinary temperatures with most common substances, while at elevated temperatures it reacts with many substances, such as titanium, aluminium, silicon, boron, beryllium, calcium, lithium or chromium, with oxygen (O2) it reacts to form oxides such as nitrous oxide (NO) and with hydrogen at high temperatures and pressure to form a very important industrial compound such as the ammonia.
Image source: Monographs.com
What are the valencies of nitrogen?
The valences of a chemical element is he number from electrons what are missing or what should they give to fill in your last electronic level.
The atoms they usually have 7 levels or layers where the electrons are located, with 1 being the innermost and 7 being the outermost. In turn, there are different sublevels, called s, p, d and f. In an atom, the electrons fill the different levels according to their energies, filling the lower energy levels first and then moving to a higher level.
To the outermost level of the atom it is also called as valence shell and the electrons located in this shell are called valence electrons. These electrons are responsible for the formation of bonds and the chemical reactions that are possible. with other atoms, that is, they are the electrons responsible for the physical and chemical properties of a element.
The different ways nitrogen combines will give it a valence (also known as the oxidation state). Nitrogen is not capable of expanding its valence shell as other elements in its group do. Its possible valences are -3, +3 and +5. The valence state of nitrogen varies depending on the compound of which it is a part. The other elements of the nitrogen family also have these oxidation states and are phosphorous (P), antimony (Sb), bismuth (Bi), moscovium (Mc) and arsenic (As).
The formation of chemical compounds with nitrogen can be explained following the valence bond theory, according to the electronic configuration of each oxidation state of nitrogen. To explain it, the number of electrons in its valence shell is taken into account and how many are missing to reach the electronic configuration of a noble gas.

Nomenclature of nitrogenous compounds.
Nitrogen compounds are chemically complex and the traditional nomenclature was not enough to name and identify them easily, so that the International Union of Pure and Applied Chemistry (IUPAC) created (also due to other factors) a systematic nomenclature in which compounds are named according to the number of atoms that form them.
This nomenclature is especially suitable for naming nitrogen oxides. Thus, nitric oxide is called nitrogen monoxide and nitrous oxide (NO), dinitrogen monoxide (N2O).
In addition to this nomenclature, in 1919, the German chemist Alfred Stock developed a method in which compounds were named depending on the oxidation state, represented in Roman numerals and in parentheses. In this way, nitric oxide would be called nitrogen oxide (II) and nitrous oxide, nitrogen oxide (I).
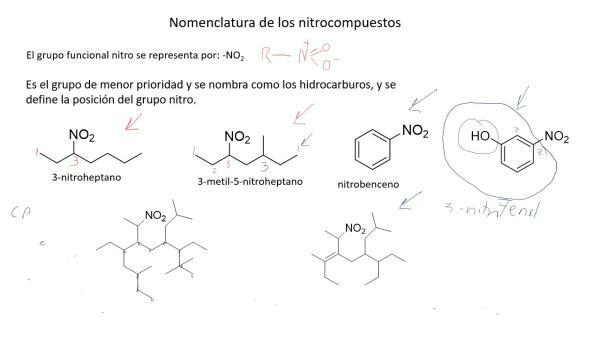
Image: Youtube
Important nitrogen compounds.
Nitrogen is capable of binding to different elements and forming a large number of compounds due to its large number of possible oxidation states. In the case of molecular nitrogen, its valence is 0 by definition.
One of the most common oxidation states is -3. In this oxidation state, nitrogen forms compounds such as ammonia (NH3), the ammonium ion (NH4-), nitriles (C≡N), imines (C=N-R) or amines (R3N). When nitrogen is in the -2 oxidation state, there are 7 electrons left in its valence shell. The odd number of electrons in its valence shell makes it easy for bridging bonds to form between two nitrogen atoms. In this state, nitrogen forms hydrazones (C=N-N-R2) and hedrazines (R2-N-N-R2). In the -1 oxidation state, 6 electrons remain in the valence shell and compounds such as hydroxyl amine (R2NOH) and azo compounds (RN=NR) are formed.
When nitrogen reaches positive oxidation states, Nitrogen bonds to oxygen atoms to form oxides, oxyacids, or oxysalts. In the +1 oxidation state, nitrogen is left with 4 electrons in its valence shell. Thus, we have examples such as dinitrogen oxide (N2O), popularly known as laughing gas, and nitrous compounds (R=NO). In the +2 state we have nitrogen oxide or nitric oxide (NO), which is a colorless gas that is generated during the reaction of metals with dilute nitric acid. This compound has a very unstable free radical that can react with oxygen to form an important atmospheric pollutant such as nitrogen dioxide (NO2)
In the +3 state, compounds such as nitrite are formed in basic solution (NO2–) or nitrous acid in acid solution (HNO2). Both are oxidizing agents that can give rise to nitric oxide (NO) or be reducing agents to form the nitrate ion. Other compounds are dinitrogen trioxide (N2O3) and the nitro group (R-NO2). In the +4 state we have nitric dioxide (NO2) or nitrogen dioxide. This is a brown colored gas that is produced by the reaction of many metals with concentrated nitric acid to form dinitrogen tetroxide (N2O4). At +5, we can find nitrates or nitric acid, which are oxidizing agents in acid solutions.
Finally, There are compounds in which nitrogen is in different oxidation states.. These are compounds such as nitrosilazide or dinitrogen trioxide.
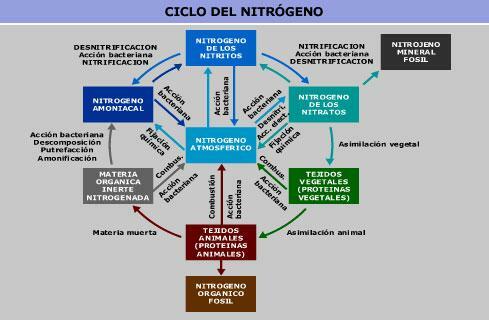
Image: Ambientum
Effects of nitrogen on health.
Molecular nitrogen is the main gaseous component of atmospheric gas. In water and soil, we can find it in the form of nitrate and nitrite. All of these compounds interconnect with each other in the nitrogen cycle.
Human action has modified the concentrations of nitrate and nitrite on land, mainly through the application of manures with nitrates on the soil. Furthermore, the concentration of nitrates and nitrites in the soil and water is increased by the nitrogen emitted by industries through the nitrogen cycle. This could also lead to increased nitrogen in drinking water.
The effects of nitrates and nitrites on human health they could be:
- Nitrates have a negative effect on the activity of the thyroid gland
- Nitrates decrease vitamin A storage
- Both nitrates and nitrites produce nitrosamines, which is a common cause of cancer
- Nitrite reacts with hemoglobin, causing a decrease in the oxygen-carrying capacity of the blood.
- Nitrogen oxide (NO) is a fundamental messenger in the human body, causing relaxation muscle, benefits in the cardiovascular system or exerting signaling effects on cells of the immune system. These effects are already exploited in multiple medicinal applications, such as medication against heart attacks or Viagra.
Environmental effects of nitrogen.
The addition of nitrates and nitrites to fertilizers causes an increase in their environmental concentrations, as well as different industrial processes. Many of these compounds can escape into the atmosphere and react with oxygen, giving rise to atmospheric pollutants that favor the increase of the greenhouse effect.
In turn, nitrates and nitrites also produce adverse effects in fresh water and in the marine environment, negatively affecting this ecosystem and the species that inhabit it. Also, the concentrations of these nitrogenous compounds in drinking water are increasing drastically, thus exerting their negative effects on human health.
If you want to read more articles similar to What are the valencies of nitrogen, we recommend that you enter our category of The atom.
Bibliography
- Mayz-Figueroa, J. (2004). Biological nitrogen fixation. UDO Agricultural Scientific Journal, 4(1), 1-20.
- Celaya-Michel, H., & Castellanos-Villegas, A. AND. (2011). Nitrogen mineralization in the soil of arid and semi-arid zones. Terra Latinoamericana, 29(3), 343-356.
- Cárdenas-Navarro, R., Sánchez-Yáñez, J. M., Farías-Rodríguez, R., & Peña-Cabriales, J. J. (2004). Nitrogen inputs in agriculture. Chapingo Magazine Horticulture Series, 10(2), 173-178.

