What are CATHODIC RAYS and their characteristics

You may not know what cathode rays are, but surely you are surrounded by devices that work thanks to them: old televisions and monitors that are currently being replaced by other technologies that allow the manufacture of flat screens and much lighter; oscilloscopes that allow us to measure signals of all kinds and that we can find in so many different places such as hospitals, mechanical workshops or recording studios... In this lesson from a PROFESSOR you will we explain what are cathode rays and their characteristics, what properties do they have and what are their main applications.
Index
- What are Cathode Rays - Easy Definition
- Discovery of cathode rays
- What are the characteristics of cathode rays?
- Where are cathode rays used? The most important applications
What are Cathode Rays - Easy Definition.
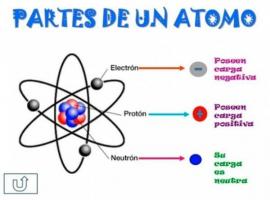
Cathode rays are currents of electrons They are emitted by the cathode (negative electrode) in a vacuum tube.
A vacuum tube It is a tube closed by valves from which almost all the gas it contained has been extracted, thus creating a space
practically devoid of atoms. The electrodes are connected to an external high voltage source that establishes a high potential difference between the two electrodes in the tube.The potential difference causes the output of electrons(subatomic particles negatively charged) from the cathode (negative electrode) to create a current that is directed towards the anode (positive electrode). This stream of electrons thus generated becomes visible in the form of a pale green glow, which originates from the cathode and is directed towards the anode.
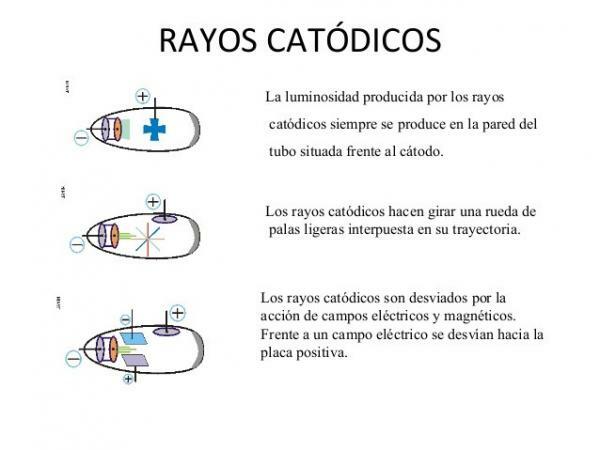
Image: 100cia.site
Discovery of cathode rays.
Cathode rays were discovered thanks to experiments carried out by William Crookes. This 19th century British scientist devised vacuum tubes of different designs to which electrodes had been incorporated.
The experiments conducted by Crookes led to the discovery of cathode rays allowed to deduce their main properties and, later, in the electron discovery. Besides the crookes tube it became a scientific research instrument that is still present in many scientific research laboratories today.
What are the characteristics of cathode rays?
Cathode ray studies using this type of light tube allow the following characteristics of this electron stream to be defined:
- Cathode rays they travel in a straight line, just like light; in the absence of electric or magnetic fields.
- They are stopped by physical barriers thick enough (like a metal plate of a few millimeters) and cast shadows in the same way that light does when it falls on opaque materials.
- The electron velocity of cathode rays increases. And by increasing the vacuum in the cathode ray tube. The greater the vacuum, the greater the intensity of the cathode rays generated. This is due to the fact that the presence of atoms in high concentrations prevents the circulation of electrons and, therefore, the emission of cathode rays. The older it is the potential difference between the two electrodes of the cathode ray tube.
- Cathode rays (currents of negatively charged electrons) they deflect when they are subjected to a magnetic field or an electric field. This is a property that does not occur in the case of light.
- Cathode rays release energy in the form of heat, since they transform their kinetic energy (energy associated with movement) into thermal energy (heat).
- Cathode rays are capable of causing somechemical reactions similar to those caused by light, such as printing on a photographic plate.
- Cathode rays ionize gases which are contained, in small quantities, in the empty tube.
Fluorescence
Another characteristic of the most important cathode rays is that they cause phenomena of fluorescence in certain materials such as glass or zinc sulphide.
Fluorescence is the ability of some materials to emit light. This phenomenon occurs when the electrons of the cathode rays collide with the material and transmit their kinetic energy to its atoms.
This energy absorbed by the atoms generates the excitation of their electrons, which jump to higher energy levels. The excited electrons quickly return to their initial position at a lower energy level. The energy released in the jump back to the initial energy state has a wavelength that is visible (fluorescence).
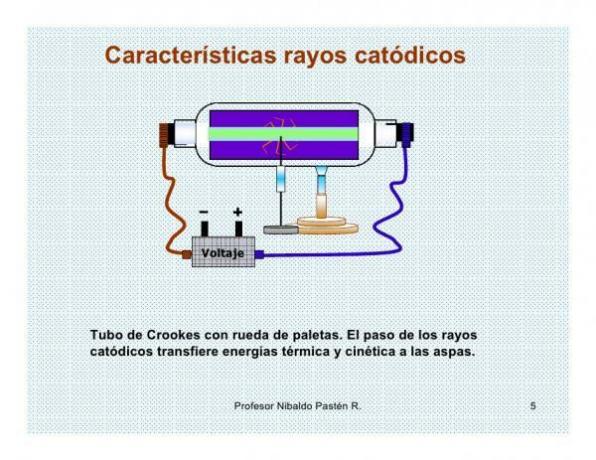
Image: Slideshare
Where are cathode rays used? The most important applications.
Cathode rays were discovered thanks to the experiments carried out by William Crookes. This 19th century British scientist devised vacuum tubes of different designs to which electrodes had been incorporated. The experiments carried out by Crookes led to the discovery of cathode rays and made it possible to deduce their main properties. In addition, the Crookes tube became a scientific research instrument that is still present in many scientific research laboratories today.
CTR (Catodic Tube Rays) technology
Today's cathode ray tube (CTR) technology is based on the vacuum tube designed by Crookes, but has incorporated some elements that allow its use in various applications.
Characteristics of current cathode ray tubes
Currently, CTRs are vacuum tubes that incorporate three fundamental elements:
- Incorporation of magnetic fields that allow to deviate the direction of the cathode rays allowing their manipulation. The incorporation of magnetic fields to divert the flow of electrons is due to the investigations carried out by J.Thomson with the Crookes vacuum tube.
- Tube coatings with fluorescent material which produces a much more intense light response because, thanks to the phenomenon of fluorescence, an important part of the cathode rays that are not visible, are transformed into light. This coating is due to the experiments of F. Braun, who experimented with the tubes of W. Crookes the phenomena of fluorescence.
- Incorporation of hot cathodes. The Crookes tube is not temperature dependent for its operation. However, T. Edison's observation that heat causes the emission of ions in some materials was applied to vacuum tubes. So-called hot cathodes were incorporated that were capable of emitting ions when heated. In this way, the operation of the vacuum tube ceases to depend on the presence of residual air inside it.
Main applications
- Measuring the velocity and mass of electrons: These properties can be measured in a CTR that incorporates an electric field and a magnetic field that cancel each other and allow the speed of the electrons and their mass to be measured.
- The oscilloscope: This device consists of a CTR that incorporates a variable magnetic field that causes a horizontal scan of the cathode rays, on the projection screen at the end of the tube. When this apparatus is connected to an instrument that measures any physical parameter and is capable of translate it into electrical signals, these are reproduced on the oscilloscope as vertical oscillations of the beam of light. It is one of the most versatile measuring instruments that exist and is used in a multitude of measurements such as: heart rate, pressure, sound levels, vibration, etc.
- Television screens and monitors: Currently CTR technology is disappearing in favor of more advanced ones, such as liquid crystal flat displays (LCD) or of emitting diodes (LEDs), which allow the size and weight of the screens to be drastically reduced in addition to having a longer useful life. However, this technology was used in TV screens and monitors until a few years ago from the 50s of the last century.
If you want to read more articles similar to What are cathode rays and their characteristics, we recommend that you enter our category of The atom.
Bibliography
Steven Weinberg (1985).Subatomic particles. Barcelona: Scientific Press S.A