Parts of an atom and their characteristics
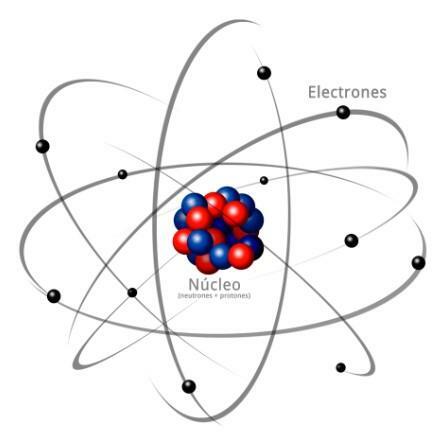
Image: Answers.tips
Atoms are the basic elements that form matter, being present in all states of matter. They are very small elements, impossible to see through the human eye but they are really important for our universe. To better understand how atoms are and how they work, in this lesson from a TEACHER we are going to talk about parts of an atom and their characteristics.
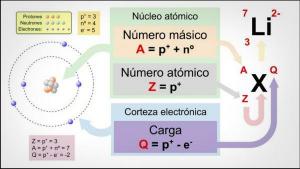
All atoms are made up of a core and a crust. The nucleus, as its name indicates, is the central part of the atom, where the particles whose charge is positive and which are called protons, and the particles whose charge is neutral, that is, they do not have an electric charge, receiving the name of neutrons. The mass of both particles, both protons and neutrons, are similar. All the atoms of the same chemical element have an equal number of protons, receiving this number the name of atomic number and using the letter Z to represent it.
On the other hand there is the Cortex what is the outer part of the atom. In the bark we find the
electrons, which are negatively charged particles. The electrons rotate at great speed around the nucleus at different levels, being particles much smaller than those that are located in the nucleus.Being neutral neutrons, positive protons and negative electrons, the atom has a neutral electric charge, since they have the same number of protons as electrons. Although there are cases in which electrons are in fewer or greater numbers than protons, causing the charge the atom is negative or positive, in this case it receives the name ion, anion if it is negative or cation if it is positive.
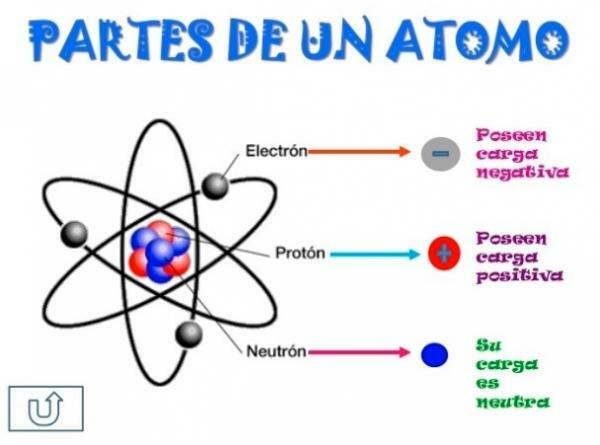
Image: Brainly
To continue with this lesson on the parts of an atom and their characteristics, we must talk about the evolution that the parts of the atom have had, since the atomic model has changed over time thanks to the studies of scientists. It must be taken into account that several of the models that we are going to explain are obsolete, not being used at present, but they are necessary to understand the evolution of the scientific community on this subject.
The historical evolution of the atomic model is characterized by following scientists:
- Dalton model: The first atomic model was the work of John Dalton in 1803. It is a very primitive model that is missing many elements such as the presence of electrons and protons.
- Thomson model: John Thomson succeeded in making a more complete atomic model than Dalton's, adding several key elements. Thomson discovered the existence of electrons and positive and negative charges.
- Nagaoka Model: The Japanese physicist Nagaoka disagreed with Thomson's model, he thought that the atom must have a large positively charged nucleus on which negatively charged electrons rotate. His theory is called Saturnian, since it compared electrons to the rings of Saturn. Many times this model is not named, but it is essential to understand the great step that is taken at this time.
- Rutherford model: Rutherford's model was based on the existence of a positively charged nucleus on which negatively charged electrons rotated. This model is very similar to that of Nagaoka, being of very close years, although the Japanese model is earlier.
- Bohr model: Bohr thought that the electrons should be far apart from the layered nucleus and the number of these orbital particles should be equal to the atomic number. His model also understands that the number of electrons varies in each shell, with fewer electrons in the first shell than in the last.
- Schrodinger model: Schrodinger broke the belief that electrons are tiny particles that revolve around the nucleus. The Austrian scientist argued that electrons moved by means of a wave function, that is, by orbital shape.
- Dirac model: Dirac modified Schrodinger's ideas for his model, using the "Dirac equation" to give a more correct view of the orbital shape of electrons.