Atom structure and characteristics

Image: SlidePlayer
Atoms are part of our world. All matter is made of atoms, so it is important to better understand what they consist of and what their functions are. In this lesson from a TEACHER we are going to tell you what the structure of an atom and characteristics. If you want to know more about the particles that make up all matter, keep reading this article.
Index
- What are atoms?
- Structure of the atom
- Main characteristics of the atom
- What are isotopes?
- How are the atoms found in nature?
What are atoms?
The atom it is the smallest unit into which matter can be divided without losing its chemical properties, that is, its properties as a chemical element. The atom is the origin of everything that can be seen or touched, from the stars to your breakfast this morning.
If we put aside the loss of its chemical properties, we can say that the atom is made up of different particles, called subatomic particles. There are three types of subatomic particles -protons, neutrons and electrons- with different characteristics.
These particles are grouped in different numbers to form the chemical elements (oxygen, carbon, etc.), but they will always be distributed following a fixed structure.

Image: Google Sites
Structure of the atom.
The structure of the atom is fixed, that is, we can have different types of atoms (the hydrogen atom, the oxygen atom, etc.) but their subatomic particles are always organized in a similar way to a planetary system.
You will surely remember how the solar system is organized: the Sun is in the center and around from this the planets rotate describing different orbits, some closer and others more distant to the Sun. In the case of atoms, in the center is the core, with a shape similar to a blackberry and is composed of the neutrons and the protons.
Around the core is the Cortex which is the area through which the electrons. Formerly it was thought that electrons described certain orbits, similar to how planets do, although now it is knows that these orbits are not so well defined and are more like areas in which we are more likely to find ourselves electrons.
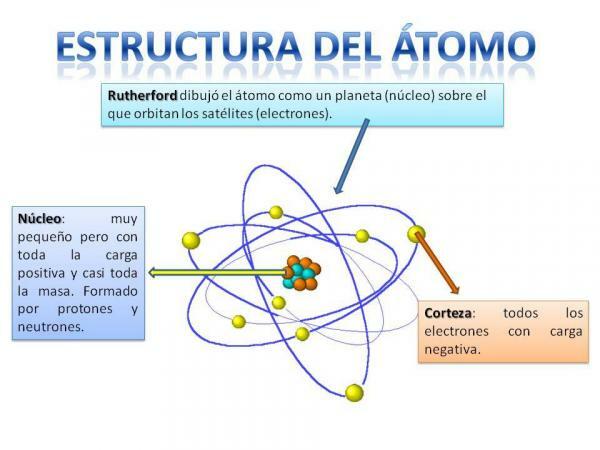
Image: REA - Plan Ceibal
Main characteristics of the atom.
To continue with this lesson on the structure of the atom and characteristics, it is important to focus on the elements that make an atom be considered as such.
And for this we have to answer this question: Why do all the atoms decide to order themselves in that certain way? The answer is simple: it is due to electrical attractive forces. The nucleus of the atom is made up of neutrons, which have no electric charge, and protons, which have a positive electric charge. and the electrons have a negative electric charge, so there is a similar attractive force between the nucleus and the electrons produced between the two poles of a magnet but is not strong enough for electrons to "fall" to the core.
This is better understood if we take into account the core size: if the atom had, for example, the dimension of a football stadium, the nucleus would have the dimension of the ball in the center from the field How much attractive force the soccer ball would have to make for the stadium bleachers to fall on it!
The function of neutrons
Now you may be wondering, and what role do neutrons play? Despite not having an electrical charge, neutrons have a great role within the atom: they contribute 99% of the mass. If it helps you remember, we can say that an atom is like a gang of three friends: the heavy neutron, the positive proton and the negative electron, which goes round and round the neutron and electron.
The movement of electrons
At this point, I want us to go back to something that we had left in the inkwell: the orbits that the electrons describe around the nucleus. We had said that electrons revolve around the nucleus as the planets do around the Sun, some closer than others, why do they do it? Electrons rotate in one shell or another according to the energy they have to move away from the nucleus, that is, those electrons that are closer to the nucleus do not have the sufficient force to move away from the nucleus while those in the outermost layers (orbitals) of the crust have more energy and have been able to move further away from the core. Furthermore, in each of the orbitals there is a maximum capacity of 8 electrons (octet rule).
The particles of an atom
We are going to return to another approach that we have done previously but have not explained: atoms are made up of neutrons, protons and electrons, which always combine following the same structure of nucleus and crust, but they do so in different numbers to form the different chemical elements. How can we get that many elements combining only 3 types of particles? The chemical elements, which you have ever seen represented in the periodic table, are characterized by having a certain atomic number.
The atomic number (Z) It indicates the number of protons in the nucleus of this type of atoms, which is equal to the number of electrons under normal conditions. Thus, for example, all atoms that have 6 protons (Z = 6) will be carbon atoms, and they will have the same chemical properties; the atoms with 5 protons (Z = 5) will be boron atoms, with the same chemical properties as each other and different from those of carbon atoms. You don't have to confuse atomic number with atomic weight or mass number (A), which is the sum of the weight of neutrons and protons (the weight of the electrons is negligible with respect to the weight of the total nucleus).

Image: SlidePlayer
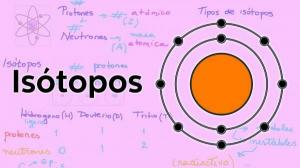
What are isotopes?
In nature we can find different "subtypes" of elements, the isotopes. I am sure that at some point you have heard of carbon 14, a radioactive isotope of carbon that is used to determine, among other things, the age of fossils. Isotopes are two atoms with the same number of protons (the same atomic number), but different number of neutrons, (different atomic mass). Isotopes of the same element are usually named with the name of the element followed by its atomic mass.
In our example, both isotopes are carbon, so they have an atomic number of 6 (Z = 6) but carbon 12 (“normal” carbon) has an atomic weight of 12 versus the atomic weight of carbon 14 14. The isotopes of the same element have very similar chemical and physical properties. In the case of carbon 14, unlike carbon 12, it is a radioactive isotope that is present in all elements that contain carbon, including living beings.
And with this we end this lesson on the structure of the atom and characteristics. We hope it has been of help to you.

Image: Your Tasks
How are the atoms found in nature?
Atoms can be found in isolation, but the most common is to find them combined in groups called molecules.
Molecules can be made up of atoms of the same element (such as the oxygen molecule, made up of two atoms of oxygen) or by groups with atoms of different elements (two atoms of hydrogen and one of oxygen form the molecule of Water). These groups of elements are called molecules, which in turn can be combined with each other and forming different more and more complex groupings according to their reactivity and their chemical affinity for source of chemical links.
If you want to read more articles similar to Atom structure and characteristics, we recommend that you enter our category of The atom.