Find out what the Millikan experiment is
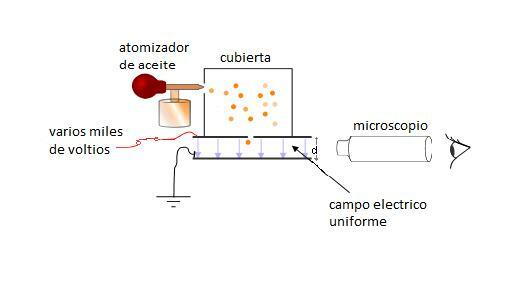
Image: Google Sites
The millikan experiment or oil drop experiment was carried out by physicist Robert Andrews Millikan and Harvey Fletcher in 1909 to determine the electron charge (although in 1911 they published an updated and improved version of the experiment initial). This experience is still considered today as one of the most beautiful experiments in physics. In this lesson from a TEACHER we will see what is the Millikan experiment Simply put, what Millikan was trying to study when he raised it and what he actually discovered. If you want to know what this elegant experiment consists of, read on!
Determine the electric charge of the electron It was vital for the development of physics since it is one of the fundamental constants of this science. Like most of the discoveries, Millikan did not discover the electric charge overnight, but he made use of knowledge and hypotheses proposed by his contemporaries.
First of all, a British physicist named J. J. Thomson in 1897 he had demonstrated the charge-mass relationship of the electron, but neither of the two separately. Thus, the researchers of the time set out to discover separately one of these two values (charge or mass), since the other could easily be calculated from the relationship discovered by Thomson.
Second, the physicists wondered if the electric charge was a continuous or discrete magnitude. A continuous quantity is one that has infinite intermediate states, such as weight or the height, that we can take as many decimal places as the instrument with which we measure allows us. In contrast, a discrete quantity is one that can be divided up to a finite limit, as by example the number of people who attend a party (it can only take integer values (twenty, fifteen, one hundred)). Some physicists, like Edison, believed that the charge on the electron should be continuous, but Millikan believed otherwise.
Therefore, the goal of Millikan's experiment was decide not only the value of the electronic charge but also that it was a discrete magnitude.
The importance of this physical constant was so great that in 1923, Millikan, won the Nobel Prize in physics, in part because of this experiment and the studies his team carried out from it and on the photoelectric effect.
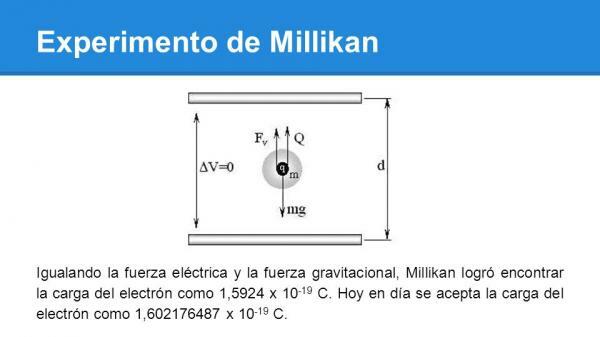
Image: Slideplayer
To begin his experiment, Millikan designed an apparatus with the following parts:
- Atomizer. This instrument allowed Millikan to obtain very small drops of oil (on the order of micrometers), necessary for his experiment to work.
- Composition chamber controlled, to avoid the dispersion of the drops and the interference of the outside air.
- Two parallel plates, one positively charged, above, and another negatively, below. The top plate has a hole for oil droplets to pass through and both are connected to a continuous power supply of several thousand volts.
- Between the two parallel plates, Millikan put a microscope to watch the small drops fall.
Millikan was running the oil through the atomizer, forming small molecules. These molecules passed into the chamber and from there to the positively charged plate. Now they had to choose a drop to work with. Thanks to X-ray irradiation, the drop became charged when it passed through the hole in the positive plate. Just as the drop reached the top of the gap formed by the two plates, the researchers adjusted the electric field until the drop remained at rest or static equilibrium. This happened when the electric force was equal and opposite to the gravitational one. This force that balanced the fall of the oil droplet is, by definition, of the same magnitude and opposite charge to the charge of the electron.
So the basic goal of this experiment was make the drop of oil float, balancing the force of gravity with the electrical forces of the applied field.
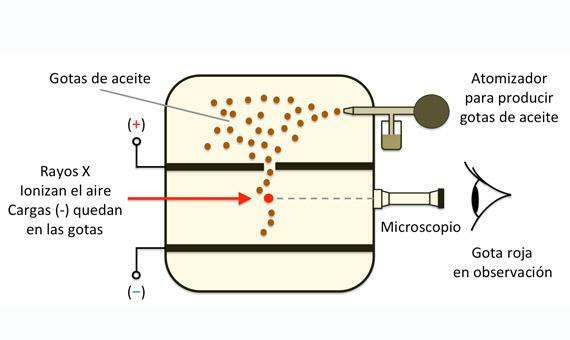
Image: BBVA
By repeating this experiment many times and with multiple drops of different sizes, Millikan and his team arrived at the result that the electric charge always takes a value equal to an integer multiple of an elementary charge: that of electron. The value obtained by Millikan and his team was 1,5924·10-19 C; this value is within one percent error of the currently accepted value, which is 1,602176487.10-19 C.
This small error is believed to be due to Millikan using the wrong viscosity value for the atmosphere inside the chamber. Furthermore, this magnitude turned out to have a discrete value, as Millikan had predicted and contrary to what he thought Edison.