Find out where the electrons are in an ATOM

Electrons are subatomic particles that revolve around the atomic nucleus in an electron cloud called electronic cortex. In this lesson from a TEACHER we will see where are the electrons within the atomic structure, as well as the behavior of electrons in the different states of the atoms (ground and arousal state), when atoms form monatomic ions and when the electrons are not bound to any particular atom.
Index
- What are electrons and where are they found?
- How do you know what the valence electrons are?
- Electrons of an atom: ground state and in state of excitation
- Electron Gain or Loss: Monatomic Ion Formation
- Electrons in motion: electric current
What are electrons and where are they found?
The electrons I know find inside of the atomswhich are the smallest units that make up matter. Atoms are indivisible and their structure and composition determine the characteristics of materials.
Atoms are made up of three types of subatomic particles:
- Protons: are particles with mass and positive charge
- Neutrons: are particles with mass and no electric charge
- Electrons: They are massless and negatively charged particles.
Protons and neutrons are part of the nucleus of the atom, so that the atomic nucleus it concentrates all the mass and the positive charge of the atom.
The electrons, on the other hand, revolve around the nucleus in defined orbits, forming an electron cloud called electronic cortex. The electronic shell of the atom concentrates all the negative charge and has no mass.
Electron orbits
The orbits that the electrons describe around the atomic nucleus are certain predefined orbits. That is, the paths of the electrons around the atomic nucleus are not random. Within the electronic shell there are only a few possible orbits for electrons; while other orbits are prohibited. is defined as atomic orbital that area around the nucleus in which the probability of finding an electron is greater than 90%.
In each of these possible orbitals, the electron that circulates through it acquires a certain energy that increases as the orbitals are further from the nucleus. The orbitals are grouped into different energy levels (n) or layers, there are a total of 7 energy levels, with n = 1 being the lowest energy level and the closest to the nucleus of the atom. In each of the energy levels or layer there are different types of orbitals (s, p, d and f orbitals)
The arrangement in which electrons are distributed in the different energy levels and orbitals in an atom is known as electronic configuration.
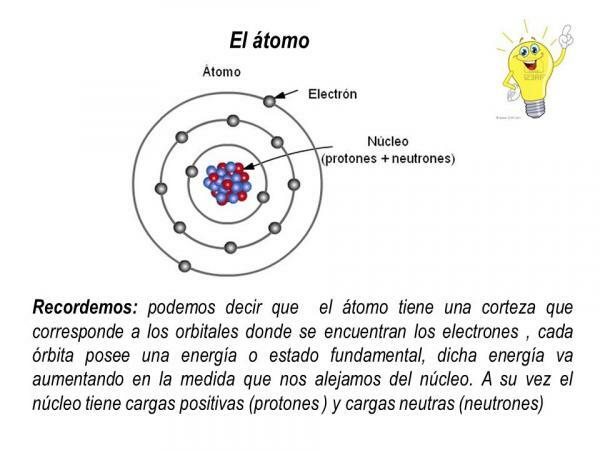
Image: Slideplayer
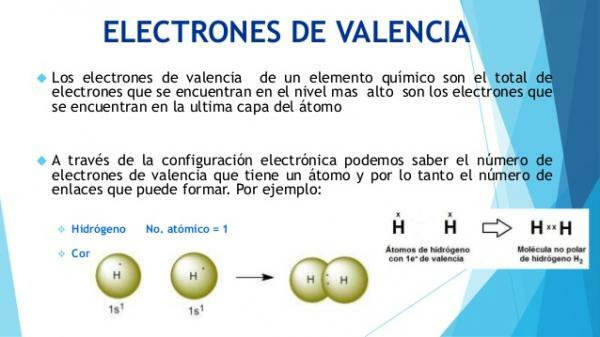
How do you know what the valence electrons are?
The physical and chemical characteristics of atoms are defined by their composition and, especially, by the electronic configuration of their outermost layer (valence layer).
Chemical elements are the different types of atoms that exist and are defined by their atomic number (Z) and their mass number (A).
- Atomic number (Z): number of protons of an atomic element, which is equal to the number of electrons if the atom is neutral.
- Mass number (A): number of particles with mass of an atomic element, that is, the sum of the particles of the atomic nucleus (protons plus neutrons).
Each element is assigned a chemical symbol that represents it and the set of all of them is consigned in the scientific document that the orders according to their atomic number and groups them according to their characteristics into families and groups of affine: Periodic table.
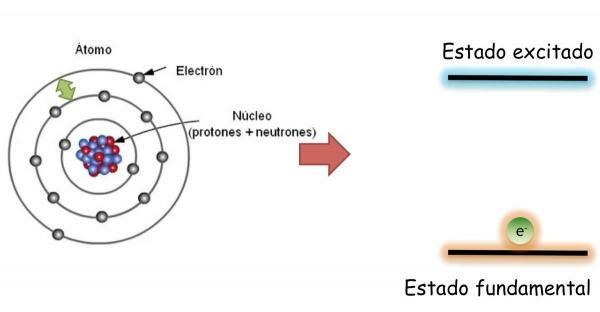
Electrons of an atom: ground state and in state of excitation.
In the fundamental state, which is defined as the state of minimum energy and maximum stability of an atom; the electrons are not distributed randomly in the different atomic orbitals, but occupy the different orbitals in an orderly way, always filling, first, lower energy free orbitals.
In this state, the atom has the same number of electrons as protons and the positive and negative charges compensate each other, therefore the atom as a whole is neutral (it has no net charge).
However, the electrons can jump from one orbital to another giving off or absorbing energy. When the electrons of an atom do not fill the orbitals in an orderly way, it is said that the atom is in excited state. In the state of excitation, one or more electrons occupy orbitals of higher energy leaving empty others of lower energy. In the excited state the atoms are highly unstable and tend to quickly return to the ground state.
When changing orbital the electron emits or absorbs energy. If the electron jumps from a lower energy orbit to a higher energy one, the atom will absorb energy; while if the jump is made in the opposite direction (from an orbital of higher energy to one of lower energy), the atom will release energy.
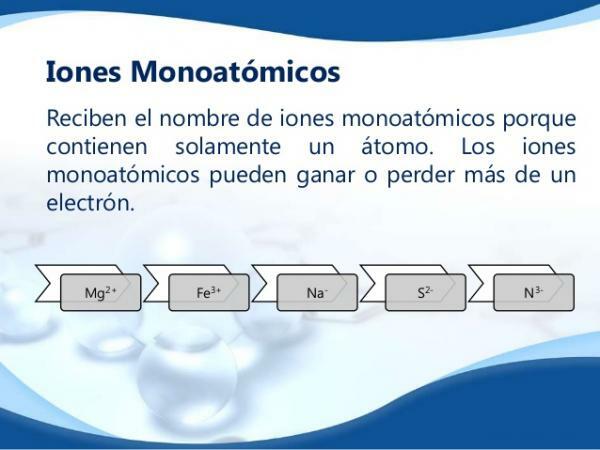
Loss or gain of electrons: formation of monatomic ions.
The electrons in the outermost shell of the electronic cortex (called the valence shell) are electrons that they can leave the atom or they can be incorporated for this one. In this way an atom can gain or lose electrons.
In a neutral atom the number of protons present in the nucleus is equal to that of the electrons that make up its electronic shell. That is, the number of positive charges is equal to the number of negative charges. When there is gain or loss of electrons, the atoms form monatomic ions.
Types of monatomic ions
According to the charge of the ion, two types are distinguished:
- Monatomic cations: atoms that have lost one or more electrons, so that part of the positive charge of the nucleus is not compensated. Therefore the atom acquires a net positive charge.
- Monatomic anions: atoms that have gained one or more electrons so that the number of electrons is greater than the number of protons in the nucleus so that the atom acquires a net negative charge.
Electrons in motion: electric current.
When electrons they are not bound to any atom in particular they move through the free space between atoms. This independent movement of electrons forms a flow of electrical charge that can travel through certain materials (conductive and semiconductor materials).
This, for example, is what happens in the case of electric current that supplies power to buildings, vehicles, etc.
If you want to read more articles similar to Where are the electrons found?, we recommend that you enter our category of The atom.
Bibliography
Alejandrina Gallego Picó, Rosa Mª Garcinuño Martínez, Mª José Morcillo Ortega, Miguel Ángel Vázquez Segura. (2018) Basic chemistry. Madrid: Uned