Characteristics of the groups of the PERIODIC TABLE
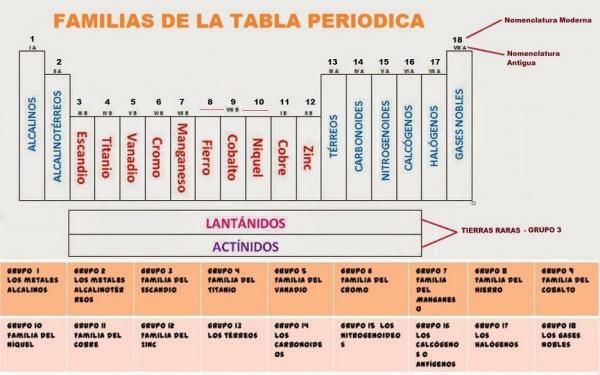
Image: Natural Sciences
The periodic table of elements order all chemical elements known so far. Although it may seem otherwise, these elements are arranged following careful rules that go from its size to its reactivity, passing through other properties such as the number of electrons in the last shell atomic.
The periodic table is, in essence, a table that is made up of rows and columns. The rows, which are arranged in the form horizontal, with the so-called periods while the columns, vertically, are the groups. But, what determines that two elements are in the same group or in a different group? In this lesson from a TEACHER we will see the characteristics of the groups of the periodic table.
Index
- What are the groups of the periodic table? How many groups are in the periodic table?
- Characteristics of group 1 (IA)
- Group 2 (IIA) characteristics
- Characteristics of groups 3 to 12 (B)
- Characteristics of group 13 (IIIA)
- Characteristics of group 14 (VAT)
- Characteristics of group 15 (VA)
- Characteristics of group 16 (VIA)
- Characteristics of group 17 (VIIA)
- Characteristics of group 18 (VIIIA)
What are the groups of the periodic table? How many groups are in the periodic table?
Before starting to talk about the characteristics of the groups in the periodic table, we have to know what this system consists of. To study the chemical elements so far discovered, Mendeleev devised this pattern in table: periodic table of elements. Horizontal rows and vertical columns appear within the periodic table. The columns of the periodic table are called groups, and elements of the same group (in the same column) have the same valence and similar chemical properties that we will see in the following sections.
Currently the periodic table is composed of 18 groups. Each of the groups is assigned a code and, in some cases, a common name.
The 18 groups of the periodic table They are:
- Group 1, IA or alkali metals. Composed of lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr)
- Group 2, II A or alkaline earth metals. Composed of Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra).
- Group 3, IIIB or Escandio family. Composed of: Scandium (Sc), Yttrium (Y), Lutetium (Lu), Lawrencio (Lr), Lanthanum (La), Actinium (Ac).
- Group 4, IV B or Titanium family. It is made up of the elements: titanium (Ti), zirconium (Zr) and hafnium (Hf) and rutherfordium (Rf)
- Group 5, VB or Vanadium Family. This group is made up of the elements: vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db).
- Group 6, VIB or chromium family. Within group 6 are included: Chromium (Cr), Molybdenum (Mo), Wolfram or Tungsten (W) and Seaborgium (Sg).
- Group 7, VIIB or manganese family. Group 7 is made up of: Manganese (Mn), Technetium (Tc), Rhenium (Re) and Bohrio (Bh).
- Group 8, VIIIB or iron family. Group 8 includes: Iron (Fe), Ruthenium (Ru), Osmium (Os) and Hassium (Hs).
- Group 9, IXB or cobalt family. Group 9 is composed of: Cobalt (Co), Rhodium (Rh), Iridium (Ir) and Meitnerium (Mt).
- Group 10, XB or Nickel family. This group is made up of: Nickel (Ni), Palladium (Pd), Platinum (Pt) and Darmstadium (Ds) (previously Ununnilio (Uun).
- Group 11, XIB, family of copper or minting metals. This group includes: Copper (Cu), Silver (Ag), Gold (Au) and Roentgenium (Rg).
- Group 12, XIIB or zinc family. Group 12 is made up of: Zinc (Zn), Cadmium (Cd), Mercury (Hg) and Copernicium (Cn).
- Group 13, IIIA or Boron family. Formed by Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl) and Nihonium (Nh).
- Group 14, IVA, carbon or carbonid family. Group 14 is formed by the elements: carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb) and phlerovium (Fl).
- Group 15, V5, family of the pnicógenos or of the nitrogenoides. It is made up of: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi) and muscovio (Mc).
- Group 16, VIA, amphogens, chalcogens or the oxygen family. Made up of: Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), Polonium (Po) and Livermorio (Lv).
- Group 17, VIIA or halogens. This group is made up of: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astate (At) and tenese (Ts).
- Group 18, VIIIA or noble gases. Group formed by: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and oganesson (Og).
Second classification of chemical elements
Within the chemical elements we can find a second classification of the groups of the periodic table.
- Groups 1, 2, 13, 14, 15, 16, 17 and 18 belong to group A of the main elements or groups
- While groups 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 belong to group B or transition metals.
Most of these chemical elements can be found in nature, either pure or in mixtures with other compounds or elements. On the other hand, some of them, like the Muscovy are artificial elements, created by man in laboratories and that have never been seen in nature. Here we discover you in more detail how the periodic table is organized.
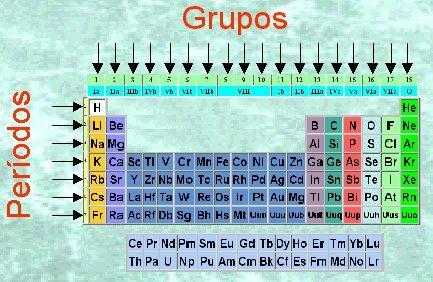
Image: Periodic table
Characteristics of group 1 (IA)
We begin by analyzing the characteristics of the groups in the periodic table, talking about the group 1 elements that have the following features:
- Oxidation number +1. They are therefore very electropositive and have low ionization energy since they easily lose this electron.
- Electronic configuration is ns1
- They are the most chemically reactive chemical elements and therefore in nature they are not isolated but in the form of salts.
- They are soft, low-density metals with low melting points. When cutting or melting them, their silver color and metallic shine are observed.
- They are malleable, ductile, and good conductors of heat and electricity.
- They form hydroxides when reacting with water

Image: Slideshare
Group 2 (IIA) characteristics.
The group 2 elements they have the following characteristics:
- Oxidation number +2
- Electronic configuration is ns2
- Low ionization energy, which becomes less and less as one descends in the group. That is why, except for beryllium, all of them form clearly ionic compounds.
- They easily react with halogens to form ionic salts.
- They have low density and are colored and soft
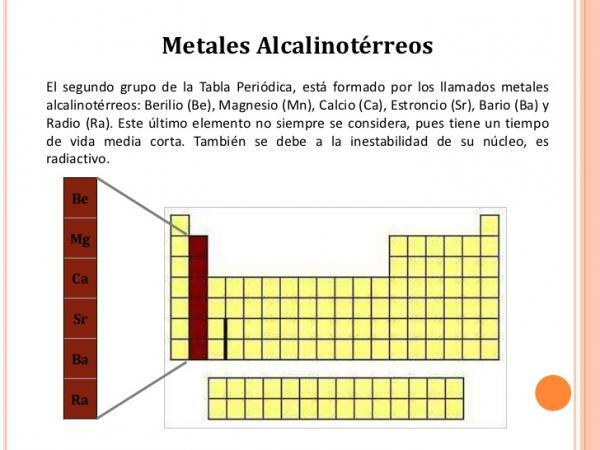
Image: Slideshare
Characteristics of groups 3 to 12 (B)
This set of groups is called transition metals or block d since, if we look at its electronic configuration, the d orbital is partially filled with electrons.
They have intermediate behaviors, that is, without being very reactive they are not very inert (little reactants), they do not have characteristic oxidation states or state, density or properties clearly defined.
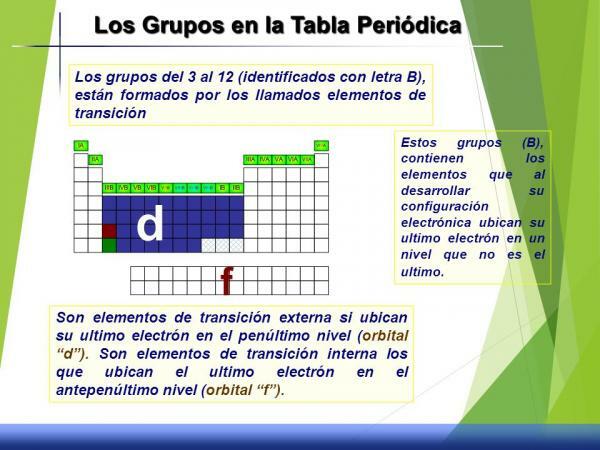
Image: Slideplayer
Characteristics of group 13 (IIIA)
Group 13Despite being also quite intermediate in terms of its characteristics, we see that it is somewhat more defined than the previous ones. Group 13 elements have:
- They have oxidation state +3 and, in certain elements, also +1
- They are usually metalloids with a very high melting point
- They tend to have typical non-metallic properties
Image: Slideshare
Characteristics of group 14 (VAT)
The carbonid elements They are also quite varied and have a very special characteristic: As we go down in the group, the elements have more metallic characteristics; carbon is a nonmetal, silicon and germanium are semimetals, and, further down the group, tin and lead are metals.

Image: Slideserve
Characteristics of group 15 (VA)
Continuing with the characteristics of the groups of the periodic table, now we will talk about the group 15 elements:
- They are very reactive at high temperature. A classic example is the reaction of nitrogen with oxygen and hydrogen, which only occurs at high temperature or pressure.
- They have 5 valence electrons.
- Covalent bonds are usually formed between N and P, ionic bonds between Sb and Bi and other elements.

Image: Slideplayer
Characteristics of group 16 (VIA)
The characteristics of the group 16 elements They are:
- They have six valence electrons (last shell s2p4)
- Its properties vary from non-metallic to metallic, as its atomic number increases.
- Its atomic volume, density, radius of the anion, and melting and boiling points increase as we move down the group.
- Its specific heat and the heat of formation of the hydride decrease as we go down the group.

Image: Slideplayer
Characteristics of group 17 (VIIA)
The characteristics of the group 17 or halogens are as follows:
- They are monovalent elements, that is, they only have one valence number. In halogens the valence is -1.
- They usually combine with metals to form Halides, Halides or Hydracids.
- They have little affinity for oxygen, so they do not form oxides but at very high temperatures.

Image: Slideplayer
Characteristics of group 18 (VIIIA)
And we end this lesson on the characteristics of the groups of the periodic table by talking about the noble gases or elements of group 18 which have the following characteristics:
- They present the state of gas in nature.
- Their electronic layer or last valence layer is complete, so they are not very reactive.
- They are very abundant in nature. After hydrogen, helium is the most abundant element in the known universe.
- They have low melting and boiling points
- They show negative electronic affinity
- Some of them are radioactive, which makes them very important from an economic point of view.

Image: Slideplayer
If you want to read more articles similar to Characteristics of the groups of the periodic table, we recommend that you enter our category of The atom.
Bibliography
- Quimicas.net (July 2015) Groups of Chemical Elements. Recovered from https://www.quimicas.net/2015/07/grupos-de-elementos-quimicos.html
- Pacheco, A (s.f) Groups of the periodic table, their description and characteristics Recovered from http://www.universidadcultural.com.mx/online/claroline/work/user_work.php? cmd = exDownload & authId = 7750 & assigId = 3 & workId = 131 & cidReset = true & cidReq = CIIS1_002
- Science Area (s.f) Periodic Table Groups Recovered from https://www.areaciencias.com/quimica/familias-de-la-tabla-periodica.html