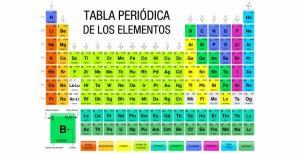
Discover how the PERIODIC TABLE is organized in an EASY and PRACTICAL way

Image: ptable.com
In 1869, the Russian chemist Dimitri Ivanovich Mendeleev devised a way to classify all chemical elements that appear in nature. This method of classification is the periodic table and many describe it as the "heart of chemistry." The periodic table was born with only 63 chemical elements, but as they were discovered, numerous chemical elements were added to its ranks.
In this lesson from a TEACHER we will review how the periodic table is organized, seeing what criteria are followed when putting the different elements in the boxes of this table.
At columns of the periodic table they have been called groups. Currently, in the periodic table that is normally used, that is, the standard one, there are 18 groups, numbered from left to right from 1 to 18. This way of naming the groups (nomenclature) can vary: sometimes a mixed nomenclature of Roman numerals and letters is used, on other occasions the groups have common names (metals alkalis, halogens, noble gases, etc.) and in others they are named as "the group of ..." and the name of its first member (for example, "the group of scandium" for the group 3).
Elements of the same group can have patterns of different properties:
- Atomic radius increase, top to bottom in a group. As we descend in the periodic table, the number of electrons increases and therefore the number of shells filled with them. Therefore, the electrons in the last shell (valence shell) are further away from the nucleus and the atoms are getting bigger and bigger, that is, they have a greater radius.
- From the top, each element has a lower ionization energy. As there are more electrons, those found in the valence shell are further away from the nucleus and therefore This attracts them with less force, making it easier to remove electrons as we go down the table. periodic.
- Finally, we also observe a decrease in electronegativity within the same group. Again, as the distance between the valence electrons and the nucleus is increasing, the electrons of other atoms are farther from the attractive force of the nucleus and therefore it attracts them less strongly than the smaller atoms (groups higher).
These regularities are trends, that is, there are certain exceptions such as what happens in group 11, where the electronegativity increases further down the group. Also, in some parts of the periodic table such as blocks d and f, the horizontal similarities between elements of the same group are not so marked.

Image: Research Library
The seven horizontal rows of the periodic table are called periods. The number of energy levels of an atom determines the period to which it belongs. Each level is divided into different categories called shells or electronic orbitals that can be of type s, p, d and f.
Like what happened in the groups, items from the same period have similar patterns atomic radius, ionization energy, electron affinity, and electronegativity:
- In a period, the atomic radius it normally goes down if we move to the right on the periodic table. As we move from one element to the next, protons and electrons are added, causing the elements to electrons are drawn into the nucleus (remember that electrons are too light for the attractive force core).
- The decrease in the atomic radius in the same period makes the ionization energy and electronegativity increases from left to right, since the attraction that the nucleus exerts on the electrons is increasing.
- The Electronic affinity it also shows a pattern over the period, albeit milder. Metals, which are to the left of the periodic table, generally have a lower affinity than nonmetals, which are to the right of the period. This is a generality and is not true for noble gases, which have their last layer (valence layer) filled and therefore are very little reactive.
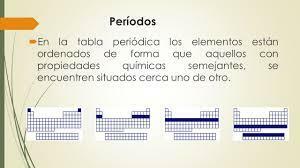
Image: SlidePlayer
The elements of the periodic table can be divided in blocks according to the order in which the electron shells of the elements are completed. Each block is named according to the latestorbital in which, in theory, is the last electron (s, p, d or f):
- The block s It is made up of the first two groups, hydrogen and helium.
- The block p It is made up of the last six groups (groups 13 to 18).
- Block d Groups 3 to 12 (commonly called transition metals) are formed.
- The block f, which is normally placed separately, below the rest of the periodic table, has no group numbers and is made up of lanthanides and actinides.
The periodic table of elements has survived for so many years because it is a system that has proven to be very useful and above all because it can be updated. In theory, there could be more elements that would fill other orbitals, but these have not yet been synthesized or have not been discovered. In the case where new atomic elements were discovered, the researchers would continue with the alphabetical order to name the different blocks (block g, block h, etc.).
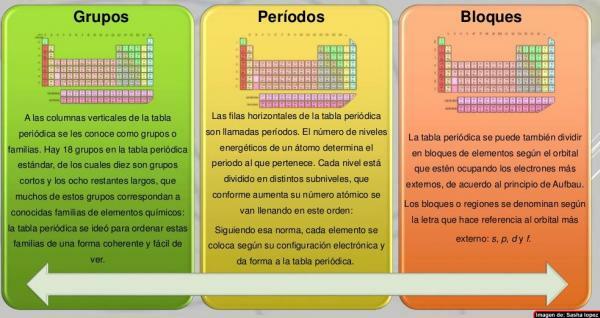
Image: Educando, the Dominican Education portal