Difference between strong and weak acids and bases (with examples)
A classification of acids and bases in chemistry depends on the ionization force of these substances in aqueous medium. A) Yes:
- a acid and a base are strong when they are completely ionized, that is, in the ionization process they are completely transformed into cations or positive ions and into anions or negative ions.
- On the other hand, an acid and a base are weak when they are partially ionized in water, that is, in solution there will be a proportion of cations, another proportion of anions and another proportion of undissociated molecules.
| Strong acids and bases | Weak acids and bases | |
|---|---|---|
| Definition | Substances that completely ionize in solution. | Substances that partially ionize in solution. |
| Ionization | Complete | Partial |
| Elements in aqueous solution | Cations and anions in the same concentration. | Cations, anions and molecules in different proportions. |
| Ionization constant | Elevated | Little |
| Examples |
|
|
Strong acids and bases
A acid or a base are strong when in an aqueous medium they dissociate completelyIn other words, the ionization process is complete and the solution will contain the same concentration of anions and cations.
How do you ionize an acid and a strong base?
A strong acid, such as hydrochloric acid HCl, ionizes in the following ways:
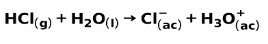
A strong base, such as sodium hydroxide NaOH, ionizes in the following ways:
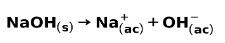
Examples of strong acids and their formulas
| Strong acids | Formula |
|---|---|
| Hydrochloric acid | HCl |
| Sulfuric acid | H2SW4 |
| Nitric acid | HNO3 |
| Hydrobromic acid | HBr |
| Perchloric acid | HClO4 |
| Chromic acid | H2CrO4 |
| Tetrafluroboric acid | HBF4 |
Examples of strong bases and their formulas
| Strong base | Formula |
|---|---|
| Sodium hydroxide | NaOH |
| Lithium hydroxide | LiOH |
| Potassium hydroxide | KOH |
| Rubidium hydroxide | RbOH |
| Cesium hydroxide | CsOH |
| Calcium hydroxide | Ca (OH)2 |
| Barium hydroxide | Ba (OH)2 |
| Strontium hydroxide | Sr (OH)2 |
| Aluminum hydroxide | Al (OH)3 |
You may be interested in seeing more examples of acids and bases.
Weak acids and bases
A Acid or base is weak when partially ionized in aqueous solution, that is, in the solution there are ions and non-ionized molecules.
How do you ionize an acid and a weak base?
A weak acid, such as acetic acid CH3COOH, is ionized in the following way:
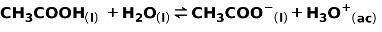
The equilibrium of this reaction is expressed by the two arrows in opposite directions.
When a weak acid dissociates or ionizes, an equilibrium is established between the species present in the solution; this can be expressed by a ionization constantsour:
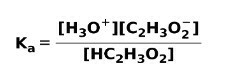
The ionization constant is nothing more than the ratio between the multiplication of the concentration of the products over the multiplication of the concentration of the reactants.
A weak base, such as NH ammonia3, is ionized in the following way:
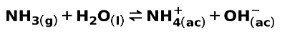
When a weak base dissociates or ionizes, an equilibrium is established between the species present in the solution; this can be expressed by a base ionization constant:
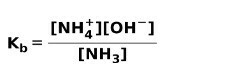
You may be interested in knowing the difference between Acids and bases.
Examples of weak acids with their formula and acid ionization constant Kto
| Weak acid | Formula | Ionization constant |
|---|---|---|
| Formic acid | H2CO2 | 1.77 x 10-4 |
| Acetic acid | H3CCOH | 1.75 x 10-5 |
| Hydrocyanic acid | HOCN | 3.30 x 10-4 |
| Hydrogen cyanide | HCN | 6.20 x 10-10 |
| Hypochlorous acid | HOCl | 3.50 x 10-8 |
| Nitrous acid | HNO2 | 4.00 x 10-4 |
| Lactic acid | HC3H5OR3 | 1.40 x 10-4 |
| Carbonic acid | H2CO3 |
4.30 x 10-7 5.60 x 10-11 |
| Boric acid | H3BO3 |
5.80 x 10-10 1.80 x 10-13 3.00 x 10-14 |
Examples of weak bases with their formula and basic ionization constant Kb
| Weak base | Formula | Ionization constant |
|---|---|---|
| Ammonia | NH3 | 1.75 x 10-5 |
| Methylamine | CH3NH2 | 4.38 x 10-4 |
| Ethylamine | C2H5NH2 | 5.60 x 10-4 |
| Aniline | C6H5NH2 | 3.80 x 10-10 |
| Pyridine | C5H5N | 1.70 x 10-9 |
| Benzylamine | C7H9N | 2.20 x 10-5 |
| Sodium bicarbonate | NaHCO | 2.00 x 10-4 |
You may be interested in knowing more about Characteristics of acids and bases