Differences between the gaseous state and plasma
Gases and plasmas are states of matter, that is, ways in which the components of matter are organized, distributed and interact in a given space.
In the case of the gases, its components are scattered trying to occupy as much space as possible. Plasma, on the other hand, is partially ionized gas.
| Gases | Plasma | |
|---|---|---|
| Definition | State of matter where atoms or molecules move freely with minimal interaction. | State of matter of ionized gases. |
| Characteristics |
|
|
| Composition | Atoms and / or molecules | Positive ions and electrons |
| Examples | Air | Ionosphere, the stars. |
What are gases?
Gases are states of matter where components (atoms or molecules) move freely with minimal interactions with each other.
A liquid can be transformed into a gas by boiling. A solid can be transformed into a gas by sublimation. An example of sublimation is when dry yarn (solid carbon dioxide) turns into gas at room temperature.
Gas characteristics
- It has no definite shape or volume.
- A gas cools as it expands.
- When a gas is compressed, its temperature increases.
- Its density is less than liquids.
Examples of gases
The atmosphere
Our planet is surrounded by a layer of gases that we know as the atmosphere. The air we breathe is a mixture of nitrogen, oxygen, carbon dioxide, argon, and other elements and compounds in a gaseous state.
Carbonic gas in carbonated drinks

Gases can dissolve in a liquid, as long as they do not react with each other (that is, they form a new compound). Thus, carbon dioxide (carbon dioxide) can dissolve in beverages, giving soft drinks that characteristic bubbly when they are opened.
Helium in balloons

When we inflate balloons with helium, a gas that is lighter than air, they can float. This is because the density of helium is 0.18 g / L while the density of air is 1.21 g / L. Helium also has the advantage that it is a non-flammable gas, that is, it does not burn.
What is plasma?
Plasma is the state of matter that results from providing energy to a gas until it ionizes. In this sense, it is made up of positively charged ions or atoms and free electrons. In the Universe, plasma is the predominant state of matter.
The creation of plasma requires energy. For example, when a gas gets hot enough that atoms start colliding with each other to the point where electrons shoot out, a plasma is formed.
The British physicist William Crookes (1832-1919) first identified plasma in 1879. The term "plasma" was assigned by Irving Langmuir in 1928 while studying ionized gases.
Characteristics of plasmas
- It has no definite shape or definite volume.
- The particles that make it up are electrically charged: negatively charged electrons, positively charged ions.
- Conduct electricity.
Examples of plasma
Although plasma is the predominant state of matter in the Universe, on a day-to-day basis it is rather rare. Let's look at some examples.
Plasma TVs
In plasma televisions, xenon or neon atoms release photons of light when excited. Some of these photons interact with phosphor materials causing them to emit visible light. Each pixel on the screen is made up of tiny pixels with different phosphor compounds for the colors blue, green, and red.
Neon lights

Neon lights are glass tubes filled with neon gas (or other gases). When electricity is passed through the signals, the electrons collide with the neon atoms, releasing their electrons and forming the Ne ion.+. The mixture of free electrons, Ne+ and neon atoms form a conductive plasma. Light is the result of electrons going from a high-energy state to a lower-energy state.
Plasma lamps
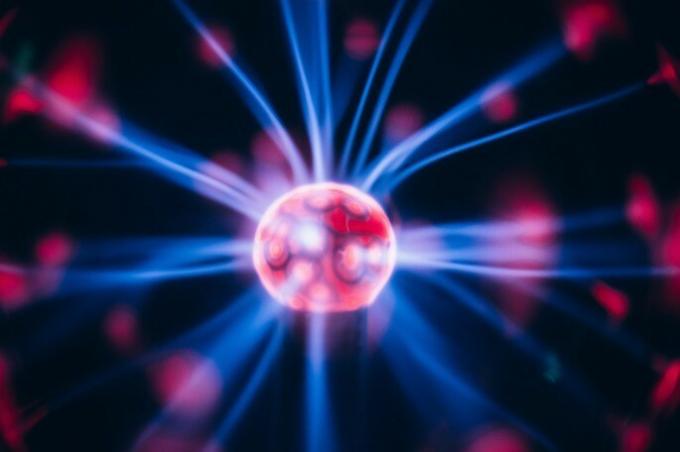
The plasma lamp was invented by Nikola Tesla when he was experimenting with high-frequency currents in a vacuum-sealed glass tube. The bright lines that we observe correspond to the phenomenon of filamentation. The colors are the consequence of the relaxation of the excited electrons to a lower energy level.
Aurora borealis

When nitrogen and oxygen atoms in the ionosphere are excited by solar radiation, electrons are released. These emit light when they return to their lowest energy state, which are visible in the northern hemisphere as aurora borealis and in the southern hemisphere as aurora australis.
You may be interested in knowing about:
- States of matter and properties of matter
- Open system, closed system and isolated system.


