What are CHEMICAL FORMULAS and what are they for [SUMMARY + VIDEOS]
Athough it does not seems, the chemical elements of the periodic table They are surrounding us on all sides. However, many times they are not found separately, but rather forming molecules or macromolecules of various chemical elements. Chemical formulas are the graphic expression of these compounds and are used in order to standardize their nomenclature. In this lesson from a TEACHER we will see what are chemical formulas and what are they for. Join us to learn more!
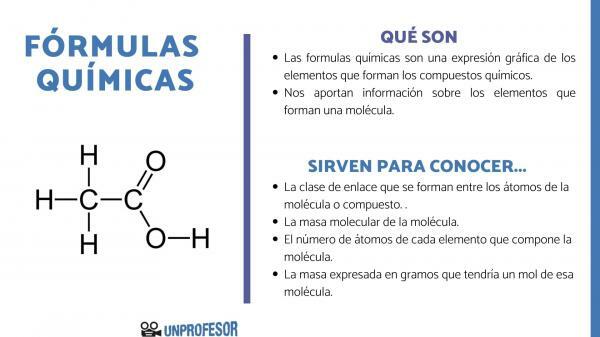
The chemical formulas are a graphic expression of the elements that make up the chemical compounds.
These chemical formulas are not arbitrary, but follow some strict rules of chemical nomenclature, established by the IUPAC (International Union of Pure and Applied Chemistry).
Chemical formulas also provide us with information about the elements that make up a molecule, thus, for example, it tells us the number and the respective proportions of each of the atoms that make it up and, sometimes, even the type of Chemical bond that exists between those elements.
Every molecule or macromolecule that exists corresponds to its chemical formula, however there are different types of formulas. Each of these types gives us different information about the molecule, but they all help us to understand its chemical nature and it helps us understand what happens in chemical reactions and how one compound can transform into others.
But in order to understand this chemical nomenclature it is necessary to have certain knowledge in chemistry, as they obey a fairly specialized technical language.

Image: Slideplayer
The chemical formulas are represented by the union of chemical symbols, with the corresponding letter of each atom as it comes in the periodic table, and subscripts, which are numbers that represent the amount of those atoms in the molecule
Within chemistry (both organic and inorganic) there are certain fragments or compounds that show a certain recurrence both structural and functional and are identified by names specific. When these fragments present unshared free electrons, they are called radicals and they are unstable, highly reactive compounds with a short half-life.
Examples of radicals are methyl groups CH3, nitrate groups NO3, hydroxyl groups OH- or the radical Cl-. However, they are known as functional groups when they are atoms or groups of atoms attached to a chain carbon dioxide (with various carbon) and which are responsible for the reactivity and chemical properties of molecules.
These groups are especially important within organic chemistry (human biomolecules are basically carbon and hydrogen). Some examples of functional groups are the carbonyl group = C = O or the carboxyl group -COOH.

Image: Monogramas.com
As we said, there are several types of chemical formulas. Each of these formulas gives us a different type of information about the molecules and, therefore, it serves a particular objective, without any formula being better or worse than another in general terms.
The classes of chemical formulas that we distinguish are:
Empirical formulas
Empirical formulas are the simplest formulas to represent a chemical molecule, sometimes being said to be minimal formulas. These formulas represent the proportion of the atoms of each of the elements in the molecule, which are simplified to whole numbers, always the smallest. An example of this formula is CH3 (methyl group), with three hydrogen atoms and one carbon atoms.
However, these formulas can sometimes give misconceptions about the composition of the molecule. This occurs when the formula does not indicate the actual number of atoms in the molecule, as with peroxide hydrogen, whose empirical formula is HO when the formula should be H2O2 (two atoms of hydrogen and two of oxygen). This thus happens through a nomenclature convention, since both subscripts are equal, they are simplified.
Some compounds, however, can only be represented by empirical formulas, since they are made up of ion networks. This occurs for example in common salt or sodium chloride, which is represented as NaCl, which indicates that for every sodium there is a chlorine.
Molecular formulas
These types of formulas are quite basic, they simply express the type of atoms and the number of each present in a covalent molecule. They are formulas that present the chemical elements and the number in a linear way (in the form of a subscript. An example of this type of formula is when glucose is named C6H12O6, which expresses that the Glucose molecule is made up of six carbon atoms, twelve hydrogen atoms and six of oxygen.
Molecular formulas are widely used, sometimes saying that they are the true formulas of molecules. In many cases, they coincide with the empirical formulas, for example in CO2.
Semi-developed formulas
Semi-developed formulas are a type of formulas similar to molecular formulas, since they also express the atoms that make up the molecule and their number, but also provide information on chemical bonds (represented by lines between atoms) and the type of bond between the carbon atoms that form it (if they are simple, double or triples). The semi-developed formula is useful for identifying the radicals that make up the molecule and its chemical structure (the bonds between the carbon and hydrogen atoms are not represented).
In the case of glucose, its semi-developed formula is CH2OH - CHOH - CHOH - CHOH -CHOH - CHO and as can be seen the atoms of carbon, hydrogen and oxygen are the same as in the molecular formula (six, twelve and six, respectively) and all single bonds (glucose only has bonds simple). This formula is therefore a more complex formula than the molecular formula.
Semi-developed formulas are sometimes called condensed and are perhaps the most widely used class of formulas. used, especially in organic chemistry, although they do not allow us to observe the real geometry of the molecules.
Developed formula
The developed formulas are a bit more complex than the semi-developed ones. In this type of formulas, the bond and the location of each atom in the molecule are represented in a Cartesian plane, until the entire compound is represented.
Structural formula
The structural formula is a graphic representation of the structure of the molecule in space, giving us information about the order and distribution of atoms in space. In this formula, the chemical bonds that make up the molecule are also shown and if they are single, double or triple. This formula is, therefore, the one that gives us the most information about the molecule.
These types of formulas are more used at a professional level within the world of chemistry, since they allow to see much more clearly the chemical reactions or synthesis of new molecules.
Lewis formulas
These are complex, very specific and technical formulas. They are also known as diagrams or Lewis structures and are similar to the developed formulas of molecules, but In addition, the electrons shared by the atoms in each chemical bond are indicated, which vary with the valence of the atoms involved.
In these formulas, the bonds between atoms are represented by lines (also indicating whether they are single, double or triple) or with a pair of dots. The solitary electrons or electrons that are not shared in the bond, are represented by points around the atom to which it corresponds.

Image: Organic Chemistry
When writing formulas, reference is sometimes made to theoxidation number of the element (it is frequent in ionic compounds). These are a set of positive and negative numbers that go associated with each element.
- The oxidation numbers can be interpreted as the number of electrons that an element shares in a covalent bond or transfers in an ionic bond.
- When the oxidation sign above the atom is negative, means that this element captures electrons and the number that accompanies it next to it is the number of electrons captured. Therefore, an oxidation state of -1 means that the element picks up one electron, -2 that picks up two, and so on.
- When the sign of oxidation is positive, the atom gives up an electron and the number that accompanies this sign is the number of electrons given up. Similarly, an oxidation state of +1 means that it gives up one electron, +2 that it gives up two, and so on.
This works mostly for ionic compounds, in covalent bonds, although the interpretation is similar, it is not the same since these bonds share electrons. In these compounds, we speak of more electronegative elements, which pull more of the electronic pair and are left with a more negative charge, depending on the number of electrons it attracts. This can be seen in the Lewis formulas.
If you want to know what chemical formulas are for, you have to take into account that this number allows us to obtain some molecule information, as they are:
- The link class that are formed between the atoms of the molecule or compound. These bonds are normally covalent when non-metallic and ionic atoms participate in it if they are metallic and non-metallic bonds.
- The molecular mass of the molecule.
- The number of atoms of each element that makes up the molecule. It is sometimes called the centesimal composition of the molecule.
- The mass expressed in grams that would have one mole of that molecule.
In the case of an ionic compound, for example sodium chloride or common salt, it is not strictly correct talk about molecules (although sometimes it is done), that these tend to form large aggregates and macromolecules. In this case, the formula of the compound helps us to see which ions form it and in what quantity.


![What are CHEMICAL FORMULAS and what are they for [SUMMARY + VIDEOS]](/f/d107af781e6392bdf80e01f34e449116.jpg?width=300&height=200)