Types of chemical FORMULAS and EXAMPLES

Chemical compounds are present in all objects and living beings of our planet. When trying to know these compounds, formulas are usually used that can be more or less complex, providing more or less information, and are called chemical formulas. In today's article a teacher We will talk about the different types of chemical formulas and examples of them. If you want to know more about them, we inform you here!
The chemical formulas are graphic expressions of the elements that are going to form the chemical compounds that exist. These formulas express the quantity and proportion of the different atoms that are part of the molecule and Some types of formulas also represent the type of chemical bond that links the different elements. To each of the known molecules or compounds there corresponds a chemical nomenclature established by the international union of pure and applied chemistry (IUPAC) according to the type of formula used.
The different types of chemical formulas that exist provide certain types of information
although in general they all serve to understand the chemical nature of the substances that form it and to express what happens during a certain chemical reaction, since sometimes some elements or compounds can be transformed into others. For this reason, chemical formulas follow a representative system of elements and molecules determined by a specialized technical language.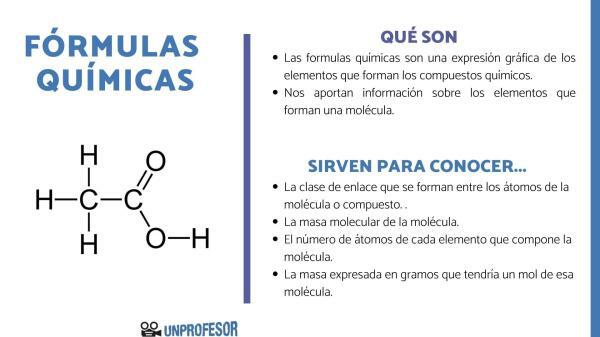
As a general rule, regardless of the types of chemical formulas, these formulas are composed of about letter-shaped chemical symbols that represent the kind of atom which forms the molecule according to periodic table of elements and some numbers in the form of a subscript next to the chemical symbol that represents its relative amount in the molecule.
In some fields of chemistry (especially in organic chemistry) there are some chemical groups that have structural and functional recurrence in molecules, which allows us to identify certain fragments of a molecule or compound. These fragments of molecules are called chemical radicals (when they have unpaired electrons) or chemical groups. functional (when they are atoms or units that cause the compound to react or behave in a certain way) manner).
Some examples of chemical radicals they are the methyl radical (CH3), the bromine radical (Br-) or the chlorine radical (Cl-). Examples of functional groups are the carbonyl group (CO), the hydroxyl group (OH) or the carboxyl group (COOH).

The different types of chemical formulas that exist use chemical symbols of the elements that compose them and the logical proportions that are established between them, which are expressed with Mathematical Symbols. The different chemical formulas used in the nomenclature are as follows.
Molecular formula
The molecular formula is the most basic formula of how many exist and expresses the type of atoms and the number of each present in the compound. The formula is very simple to write and interpret, it simply presents the symbols of the chemical elements and their numbers in the form of subscripts in the form of a linear sequence. For example, we have the following formulas:
- Basic oxides: Na2O (sodium oxide), K2O (potassium oxide), MgO (magnesium oxide).
- Hydroxides: NaOH (sodium hydroxide) KOH (potassium hydroxide), Ca (OH)2 (calcium hydroxide), Mg (OH)2 (magnesium hydroxide)
- Acids: HCl (hydrochloric acid), H3PO4 (phosphoric acid), H2SO3 (sulfurous acid), H2SO4 (sulfuric acid)
- Salts: NaCl (sodium chloride), Na2SO3 (sodium sulfite), Na2SO4 (sodium sulfate)
semi-developed formula
The semi-evolved formula is similar to the molecular formula and expresses the atoms that make up the compound, the chemical bonds in the form of lines and what type of bonds are those that link the atoms of the compound (simple, double or triple). However, in this type of formula carbon-hydrogen bonds are not represented. These types of formulas are widely used in organic chemistry nomenclature. Examples of these types of formulas are:
- Semi-developed formulas in organic chemistry: CH3-CH3 (ethane), CH3-CH2-OH (ethanol)
- Semi-developed formulas in inorganic chemistry: they are rare, some are PO(OH)3 (phosphoric acid), (OH)2P(O)OP(O)(OH)2 (pyrophosphoric acid)
developed formula
This is another of the types of chemical formulas. It is a little more complex than the semi-developed. In this type of formula, the bond and also the position of the atom within the molecule are indicated, representing the structure of the compound in a Cartesian plane.
structural formula
This type of chemical formula is very complex, but it provides a great deal of information about the molecules, which represents, in addition to its structure and organization, its spatial form in a bi or three-dimensional.
Lewis formula
They are also known as Lewis diagrams or Lewis structures and are similar to the developed formulas, but in this case also the electrons shared in each bond between atoms are represented, which are represented by points attached to the lines of the links. Unshared electrons are also represented as dots on the atoms. These formulas are very technical and are used in very specific fields.



![What are CHEMICAL FORMULAS and what are they for [SUMMARY + VIDEOS]](/f/d107af781e6392bdf80e01f34e449116.jpg?width=300&height=200)