How to get the MASSIC NUMBER?

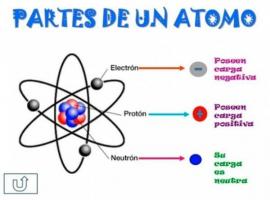
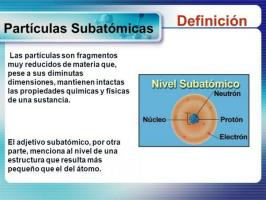
Atoms are part of all the matter that makes up the universe. Matter is very varied since it is made up of different types of atoms, with different characteristics and properties. The characteristics of the different atoms are given by the particles that form them: protons, electrons and neutrons. The different proportions between one and the other are defined in chemistry with different names (mass number, atomic number, etc.). The mass number gives us the final or total number of particles in the nucleus, or what is the same, it is make the sum between protons and neutrons, and is used in chemistry to differentiate the isotopes of an element chemical.
In this lesson from a TEACHER we will see the mass number, how to get it, what exactly it is and what it is used for.
Index
- What is the mass number?
- The mass number and isotopes
- How to calculate the mass number? - Formula
What is the mass number?
The mass number it is nothing more than the sum of protons and neutrons
, that is, all the particles that are in the nucleus (remember that the electrons are orbiting around the nucleus, forming the crust).The mass number is written with the letter a and it is indicated in a superscript situation, always to the left of the symbol that indicates the element we are dealing with, it is that is, we will find it as a small number that is placed to the left of the element symbol, at the top of this. It represents the mass of the atom measured in u.m.a (atomic mass unit) because the mass of the electrons is really small, it is so small that it can be ignored or neglected.
The mass number of atoms is normally used to differentiate isotopes of a chemical element.

Image: Slideshare
The mass number and isotopes.
Isotopes are variants of the same chemical element that have different amounts of neutrons so differ in mass number. Isotopes can occur normally in nature (natural isotopes) or be entirely man-made (artificial isotopes). Examples of isotopes in nature are those of carbon:
Carbon occurs as a mixture of three isotopes with mass numbers 12, 13, and 14: 12C, 13C and 14C.
Isotopes have numerous applications: they are used for cancer treatments, determining the appearance of poisons in tissues such as arsenic, markers of chemical reactions, etc.
Each of the isotopes of a chemical element can have different characteristics. One of the best known and most used characteristics is the constant half-life of half-life or half-life. The half-life of an isotope is the time required for half of the nuclei in an initial sample of a radioisotope to disintegrate. In practice, isotopes tend to disintegrate, changing from less stable forms to more stable forms. stable, so this can also be understood as the time it takes to transmute or transform the half of the atoms radioactive from a sample. The half-life of carbon-14, which becomes carbon-12, is very long and is used to date ancient organic remains such as fossils. In contrast, the half-life of other isotopes such as oxygen-15 is only seconds (122 seconds, to be exact).
In the case of hydrogen, its natural isotopes they have very different characteristics and properties, which is why they have three different names: conventional hydrogen or protium 1H, deuterium 2H (D) and tritium 3H (T). Hydrogen has other artificial isotopes (hydrogen-4, hydrogen-5, etc.).
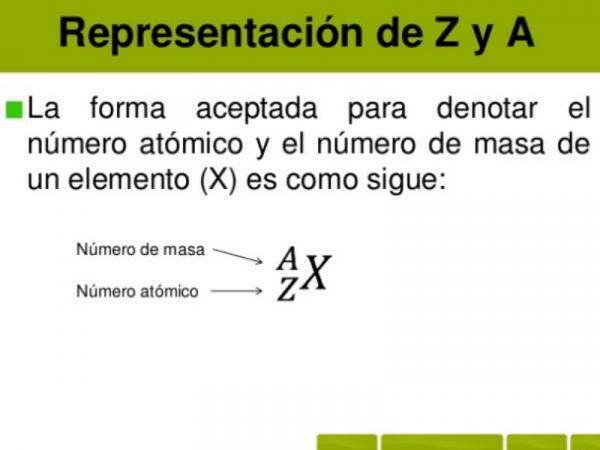
Image: Slideshare
How to calculate the mass number? - Formula.
In order to calculate the mass number of an atom or ion we will have to add the atomic number (number of protons in an atom or ion, normally represented as "Z") and the number of electrons.
Mass number (A) = atomic number (Z) + number of neutrons (N)
TO = Z + N
The atomic number can be found in the periodic table of the elements, in the upper left of each of the elements. Also, the mass number or atomic mass will appear below the element in the periodic table.
Therefore, by subtracting the atomic number from the mass number, we can also know the number of protons.
Number of neutrons (N) = mass number (A) - atomic number (Z)
N = A - Z
For example, if you consult a periodic table you will see that iron has a mass number of 55.84, that is, A = 56 rounded to the nearest unit; Its atomic number (Z) is 26, so its number of neutrons will be:
N = A - Z = 56-26 = 30
If you want to read more articles similar to How to get the mass number?, we recommend that you enter our category of The atom.