What are ISOTOPES for?
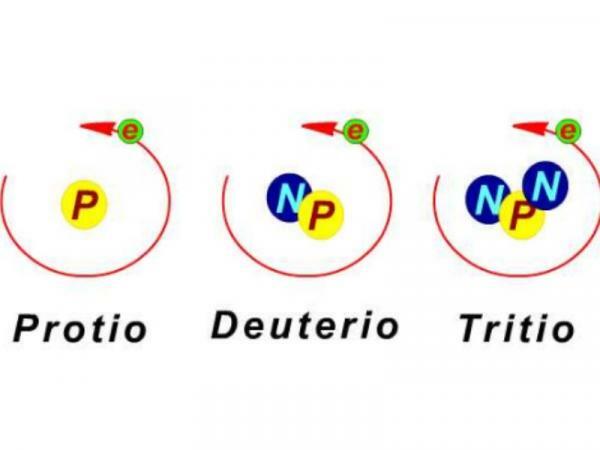
Isotopes are atoms of the same element that have the same number of protons in the nucleus but have different numbers of neutrons. This difference that, a priori, it may seem quite insignificant, it makes the isotopes of the same element the same or different in many characteristics and properties, physical and chemical. Human beings have been able to take advantage of all of them for our benefit. The applications of isotopes are varied and affect very different fields: they help us to conserve our food, to diagnose and cure diseases or to find out the age of fossil remains buried millions ago of years. In this lesson from a TEACHER we will see what are isotopes for and how they help us in ten different areas of our life.
Index
- What are isotopes?
- Isotopes and obtaining energy
- Isotopes in medicine
- Isotopes in art and archeology
- Isotopes in food and agriculture
- Other applications of isotopes
What are isotopes?
The atoms They are made up, in a classical way, of three types of particles:
neutrons, protons and electrons. The neutrons and protons form the nucleus while the electrons revolve around the nucleus, forming an "electron cloud".The atoms of a chemical element are characterized by having a certain number of protons. The number of protons of an element in chemistry is called atomic number (Z). Atoms are electrically neutral, that is, they have the same total number of positive particles (protons) as negative (electrons). Another thing that characterizes atoms is their mass number (A) which is the sum of all the protons and neutrons that make up the atomic nucleus and that give it most of its weight or mass. You can learn more about it in this other lesson from a TEACHER on How to get the mass number?
However, two atoms of the same chemical element (with the same atomic number) can have different numbers of neutrons in their nucleus, and therefore different atomic weight. These two atoms, with the same atomic number but different atomic weights, are called isotopes. Isotopes of the same chemical element may have characteristics or properties that make them very interesting and useful for human beings. If you want to know more about the subject you can visit our lesson Characteristics of isotopes.
Now that you know what they are, if you have ever wondered what are isotopes for, keep reading!
Isotopes and obtaining energy.
As you know, one of the main sources of energy on the planet is the nuclear energy. The name of nuclear energy comes from the fact that energy is obtained, literally, from the nucleus of atoms. As we saw earlier, the nucleus of atoms is made up of protons and neutrons, which are linked together due to a great force. This energy can be released and used by man through two processes: nuclear fusion (the combination of two nuclei, which join to form a larger and more energetic one) and the Nuclear fision (the disintegration or separation of a nucleus into several smaller ones).
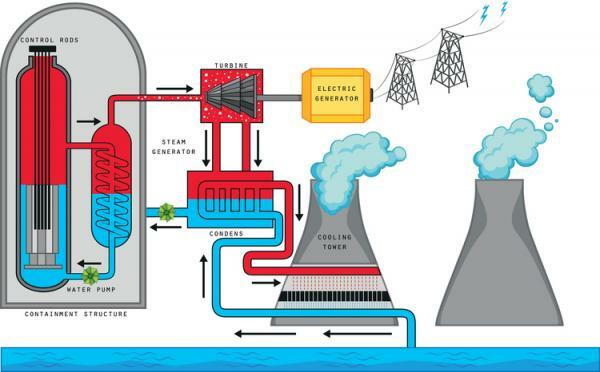
Currently, most of the production of nuclear energy is done by nuclear fission. For this, the Plutonium isotope «Pu-239» and Uranium isotope «U-235» since they are very unstable and heavy and the division of the nucleus entails a fairly high energy production. When the nuclei of these isotopes rupture, a large amount is generated in the form of heat; This heat is used in nuclear reactors to heat water, which goes from a liquid state to a gas. This water vapor is transported to a turbine, which moves. In turn, that turbine is connected to a Electric generator whose mission is to transform the turning energy of the turbine into electrical energy, which can be stored, transported and used in our homes.
Isotopes in medicine.
One of the fields in which the application of isotopes is greatest is in medicine. We can distinguish two phases during the disease in which isotopes are used: diagnosis and treatment of the disease.
For the diagnosis of diseases and pathologies, techniques such as x-rays or scintigraphy; the scans are based on a method of detecting radiopharmaceuticals, which are nothing more than mixtures of substances among which there are small amounts of a radioisotope. They can also be used to determine small amounts of enzymes or proteins generated in some biological processes.
Isotopes can be used to treat diseases. Isotopes are used during radiation therapy treatments to destroy cells that form malignant tissues and tumors; In this process, the ionizing radiation from medical equipment reaches the tumor cells and affects their DNA, generating so many breaks and mutations that they cause their death. In addition, before applying radiotherapy treatments, isotopes can help doctors study the characteristics of tumor cells, their location, etc. and in this way create an action plan to adjust the dose, frequency and number of irradiation sessions necessary before starting the treatment.
Another application of isotopes in medicine is the application of radioisotopes for the sterilization of material, equipment and facilities such as operating rooms. There are different types of radiation sterilization depending on the element we use, although the most widely used is ultraviolet (UV) radiation.
Isotopes in art and archeology.
If you have ever wondered what isotopes are for in the world of art and archeology, you may have come across one of their applications: dating of works. The isotopes and the comparison of the half-life of these allow to date the age of samples archaeological sites from excavations but also from works of art such as paintings, murals or even sculptures. Another of the most relevant applications in art is the identification of forgeries or alterations of works: thanks to isotope analysis it is possible to know not only the date on which a work was made (and detect possible copies) but also to know what materials were used to make it (and compare them with those used by the author of the supposed work), you can investigate the inner layers of the works without damage them, etc. Microorganisms and insects that were trapped in wet paintings can even be detected and their age analyzed.
Finally, isotopes can also be used to preserve works and prevent them from being damaged. Certain works can be covered in a substance to which gamma radiation is then applied. This combination of monomers and radiation slows down the deterioration of the work since it reduces the progressive loss of fixation that the work suffers when exposed to the environment, it is sterilized and insects, fungi and others that damage and contaminate the plays. This method is usually applied to paintings but there are other methods that allow to recover even books in extreme conditions of conservation.

Isotopes in agriculture and food.
Agriculture and food use isotopes at all levels, from before the plant is planted until it reaches our table. Isotopes are used for the genetic improvement of plant species that are very important for human consumption, which allows the development of genetic varieties more productive, resistant and efficient. Once in the plantation, the isotopes allow us a conscious use of fertilizers since they allow us to detect their overuse. Finally, during their preparation for marketing, isotopes are used during sterilization both of containers as the same product itself, making it last longer and in better conditions, until its consumption.
Other applications of isotopes.
There are many other applications of isotopes, such as the following:
- Study of the water cycle. Hydrology uses isotopes to follow the water cycle and detect possible contamination of the water during different phases of the cycle. One of the most important applications is the detection of groundwater contamination, especially from the overuse of fertilizers.
- Pollution studies. Isotopes are not only used to determine water contamination as we have seen in the previous point, but they can be used to determine the presence of compounds sulfur and nitrogen pollutants in the atmosphere or to study the contamination of sediments by petroleum derivatives that can come, for example, from a spill illegal.
- Applications of isotopes in industry. The study and knowledge of the properties of the different isotopes have allowed the industry to improve and develop processes but also to verify that different instruments, parts or objects are in perfect condition (control of quality).
- In mining and cosmology. Isotopes are used to create nuclear probes, in charge of studying the composition of the different layers that make up our planet. In contrast, the study of the isotopes that contain meteorites allows us to investigate the age of the universe, the possible origin of these meteorites and its characteristics.
- Isotopes and the study of outer space. Isotopes are used to make nuclear cells; These devices are used as energy stores that power the equipment and instruments of satellites and space probes.
If you want to read more articles similar to What are isotopes for?, we recommend that you enter our category of The atom.
Bibliography
- Guerrero, R., & Berlanga, M. (2000). Stable isotopes: Rationale and applications. Current Spanish Society of Microbiology. (29).
- Enresa-UCO Chair (s.f) Use of Radioactivity.
